In June we will be joining elmi2021, an annual meeting, which will be held virtually this year from the 22nd to the 25th of June. During two Delmic workshops, we will highlight our recent developments in the fields of cryo-electron tomography and high throughput electron microscopy. Make sure to reserve your spot if you are joining the meeting!
Workshop: Integrated correlative light and electron microscopy (CLEM) to streamline the cryo-electron tomography (cryo-ET) workflow
Cryo-electron tomography (cryo-ET) is a powerful technique to acquire high-resolution 3D structures such as intracellular organelles and protein complexes in their near-native cellular environment. The current sample preparation method can be challenging as it often requires cryo-focussed ion beam (FIB) milling to create an electron transparent lamella and CLEM to ensure the region of interest (ROI) is present in the lamella. To simplify the workflow we introduced METEOR: a fluorescent light microscope that can be retrofitted to a (cryo) FIB/SEM.
In this workshop, our speakers Caspar Jonker and Marit Smeets will show how our integrated cryo-CLEM systems were used to image a variety of cells, such as yeast and HeLa cells. We show that the fluorescence data could also be used to target the ROI inside the cells and confirm its presence during and after milling. By employing cryo-ET on FIB milled lamellae the inside of the cells were visualized with a high resolution.
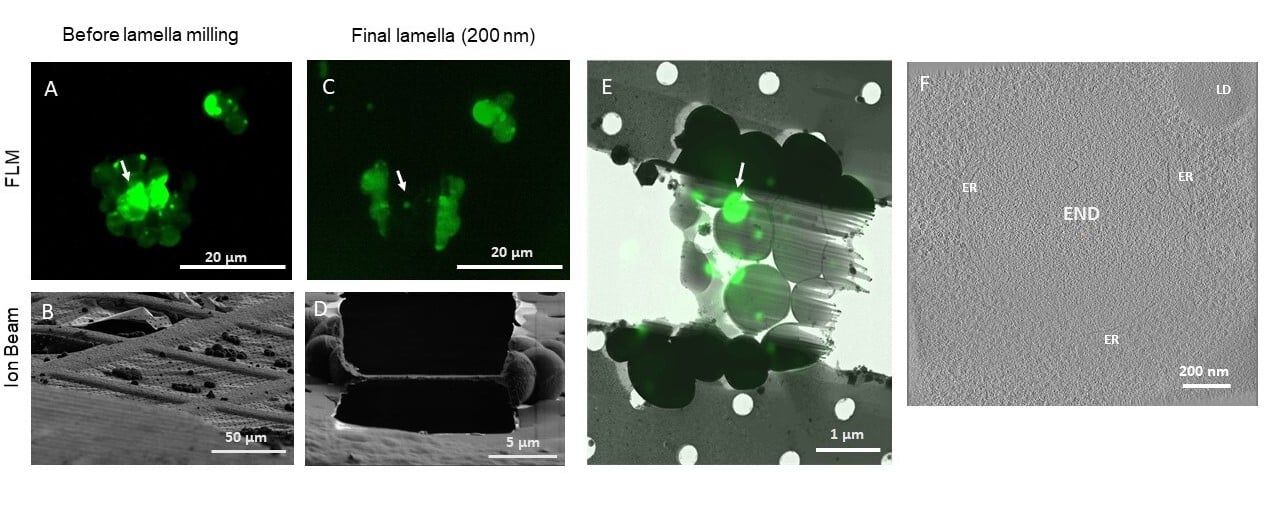
Figure 1: Correlative cryo-FIB milling and cryo-ET workflow using METEOR, demonstrated on a sample of S. cerevisiae expressing eGFP-Ede1 A-D: Maximum intensity projections (MIPs) of fluorescence Z-stacks (A,C) and ion beam views (B,D) of a lamella before milling (A,B) and after fine milling (C,D). Fluorescence Z-stacks were acquired with excitation at 484 nm, an emission filter 525/30 nm and a z step size of 400 nm. E: Overlay of the final lamella MIP on the TEM lamella overview. Arrows in A, C and E indicate the targeted fluorescent punctum where the tomogram in (F) was taken. F: A 2D slice of a tomogram acquired on the indicated position on the lamella. The target structure, a phase separated END surrounded by fenestrated ER, can be clearly distinguished from the surrounding cytosol containing ribosomes. LD: lipid droplet. Images courtesy of Anna Bieber, Cristina Capitanio and Oda Schioetz, MPI Biochemistry, Martinsried, Germany.
Join this workshop if you would like to better understand how METEOR can be used in the cryo workshop. We will demonstrate how to navigate the sample to find an ROI, how to take high-quality images and various approaches to correlate your data, all within the SEM chamber.
Time and date: 23rd of June at 12:15 (CEST).
If you would like to join this workshop, please register here.
Workshop: Supplementing light microscopy with high-throughput EM
Combining electron microscopy (EM) with light microscopy (LM) for life science applications can have substantial benefits. Such combination could provide the benefits of understanding the structure underlying changed behavior or composition, as observed by FM.
However, until today EM has seen limited use for large-scale investigations due to a very limited throughput. During this workshop our speaker Job Fermie will present Delmic’s FAST-EM multibeam system, which provides a significant improvement in imaging throughput over EM workflows and enables a shift towards using EM as a faster, quantitative analysis tool. FAST-EM, and high-throughput EM in general, will be instrumental in tackling current large-scale projects such as volume-EM of cells and tissues, connectomics, and large-scale 2D nanotomy projects.

Figure 1: Multibeam STEM imaging on 0.25mmx0.25mm area of rat pancreas within 5 minutes.80 nm ultrathin sections of OTO-stained, Epon-embedded pancreas tissue on scintillators was imaged at 5kV landing energy, 4 nm pixel size, 3200 ns dwell time. (A) Typical image collected in multibeam STEM. Acquisition of a single field with the 64-beam requires 3 seconds and covers 25.6×25.6μm. (B) The images collected by individual beams, which each scan 3.2×3.2μm. The resolution allows identification of membrane systems, organelles and macromolecular complexes (right panel). (C) Megafield of the pancreas tissue, consisting of 100 multibeam images, acquired in less than 5 minutes while retaining the high image quality shown in (A) and (B).
Additional benefits of high throughput are seen in correlative light and electron microscopy workflows. In traditional CLEM workflows, EM is a limiting factor for the scope of data collected: with low throughput and limited field of view, careful selection of ROIs is needed to ensure the feasibility of a project. Combined with high-throughput EM, however, larger volumes can be investigated by CLEM, enabling a transformation towards integration of imaging modalities on a large scale. With FAST-EM, CLEM could be used as a holistic tool to integrate data on dynamics, composition and ultrastructure.
Time and date: 24th of June at 12:00 (CEST).
If you would like to join this workshop, please register here.
The European Light Microscopy Initiative was created in 2001 to establish a unique communication network between European scientists working in the field of light microscopy and the manufacturers of their equipment. Its aim is to promote the quickly developing field of light microscopy as a fundamental research tool for the life sciences and to strengthen the channels of communication between researchers, core facilities and industry. Learn more about it on the official website.
.png)






