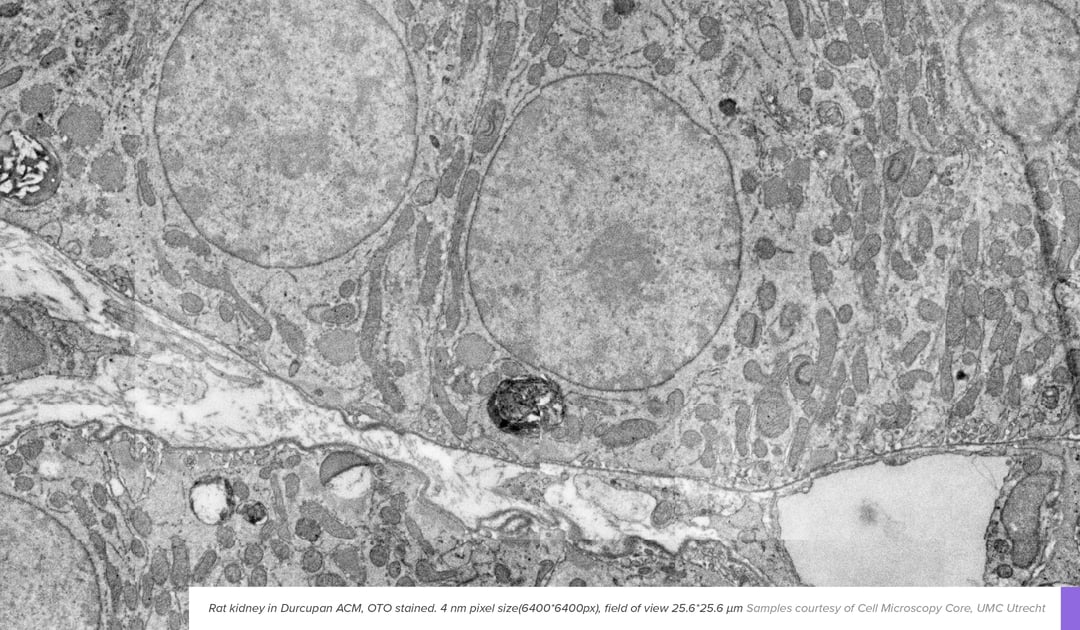
In EM, contrast is generated when electrons interact with the sample. However, most biological material consists of light elements such as carbon, oxygen, and nitrogen, which interact with the electron beam only sparsely. To enhance the contrast of specimens and enable room-temperature EM imaging, contrasting agents are introduced. These react with the components that build up cells, like lipids, proteins, and nucleic acids, causing enhanced visibility of these components.
Basic protocols using some combination of incubation steps with osmium, uranyl or lead salts usually provide enough contrast for routine experiments. Depending on the mechanism of action they are added prior to embedding (osmium, uranyl) or after embedding and sectioning (lead citrate). Although parameters like concentrations and incubation length vary based on experience and preference, osmium, uranyl and lead stainings have been the backbone of electron microscopy experiments for the last few decades. However, adaptations are still made to match new applications and EM systems, which can provide substantial benefits to throughput, or enable new applications.
Enhanced staining protocols
Enhanced staining protocols are typically tailored to a specific problem seen in an application. As an example, in applications where high throughput imaging is essential, staining protocols are tuned to bind significantly more heavy metal to biological structures. The higher metal content in the sample improves contrast generation, which in turn means less electrons are needed to create an image with adequate signal. Among the most common tools for contrast enhancement is a compound known as thiocarbohydrazide (TCH). TCH enhances membrane visibility by binding to osmium tetroxide, which provides more sites for osmium tetroxide in subsequent steps [1]. Other chemicals can also be employed for similar effects, enhancing the level of penetration or level of deposition of heavy metal salts [2]. Such contrast enhancement protocols work great in the fields like connectomics, where the higher penetration depth is beneficial considering large sample sizes, and the enhanced contrast aids in boosting throughput [3].
Mild staining protocols
Although using mild staining protocols seem counterintuitive from a throughput perspective, there are situations where reducing the level of contrasting is essential, one of them being in correlative light and electron microscopy (CLEM). Here, fluorescence preservation is an essential part of the workflow, which can be challenging considering some of the effects of heavy metal salts.
Osmium tetroxide is known to irreversibly damage fluorophores by chemical crosslinking, while uranyl and lead are known to quench fluorescence. Minimizing their disruptive effects while retaining contrast therefore requires careful selection of heavy metal contents during preparation. As a result, mild staining protocols using only uranyl acetate have become prevalent in CLEM workflows [4], where the compromise of reduced contrast is offset by the information of fluorescence microscopy.
While selecting the right staining technique plays a big part in the workflow, having a flexible and fast microscope is what really allows to easily produce good quality images with any samples. With our FAST-EM system, our new multibeam electron microscope, you can image a wide range of samples and work on as many projects as possible within the same system. The reliability and speed that FAST-EM offers allows you to spend more on other steps of the workflow: preparing the samples, analyzing the data and making conclusions.
Join our webinar 'Next generation electron microscopy with FAST-EM' to see FAST-EM in action. During the webinar our applications specialist Job Fermie will go through all the steps that are required for capturing large-scale data, including:
sample preparation and loading, image acquisition and browsing through acquired data.
References
[1] J. C. Tapia et al., “High-contrast en bloc staining of neuronal tissue for field emission scanning electron microscopy,” Nat. Protoc., vol. 7, no. 2, pp. 193–206, 2012.
[2] C. E. Thoeni et al., “Microvillus inclusion disease: loss of Myosin vb disrupts intracellular traffic and cell polarity.,” Traffic, vol. 15, no. 1, pp. 22–42, Jan. 2014.
[3] C. S. Xu et al., “Enhanced FIB-SEM systems for large-volume 3D imaging,” Elife, vol. 6, pp. 1–36, 2017.
[4] W. Kukulski, M. Schorb, S. Welsch, A. Picco, M. Kaksonen, and J. A. G. Briggs, “Correlated fluorescence and 3D electron microscopy with high sensitivity and spatial precision,” J. Cell Biol., vol. 192, no. 1, pp. 111–119, Jan. 2011.
.png)