Luminescence is present all around us in daily life. It is the spontaneous emission of light by a substance when it is not resulting from heat. It can be caused, for example, by chemical reactions, electrical energy, subatomic motion, or stress on a crystal. There are different types of luminescence:
- Chemiluminescence (emission of light as a result of a chemical reaction)
- Bioluminescence (the result of a biochemical reaction taking place inside a living organism)
- Photoluminescence (a result of a material absorbing photons)
- Electroluminescence (excited by an electric current flowing through a material)
- Cathodoluminescence is a specific form of luminescence caused by ‘free’ electrons (or simply electrons propagating through space). The name comes from the times when electrons used to be called cathode rays.
Why is CL so interesting and why would we want to look at it? Cathodoluminescence is a visible measure of what is taking place inside a material or an organism. When looking at materials with conventional methods, such as optical microscopy, it is impossible to achieve the same spatial resolution as with electron microscopy. Cathodoluminescence is a great tool for looking at light with very high spatial resolution.
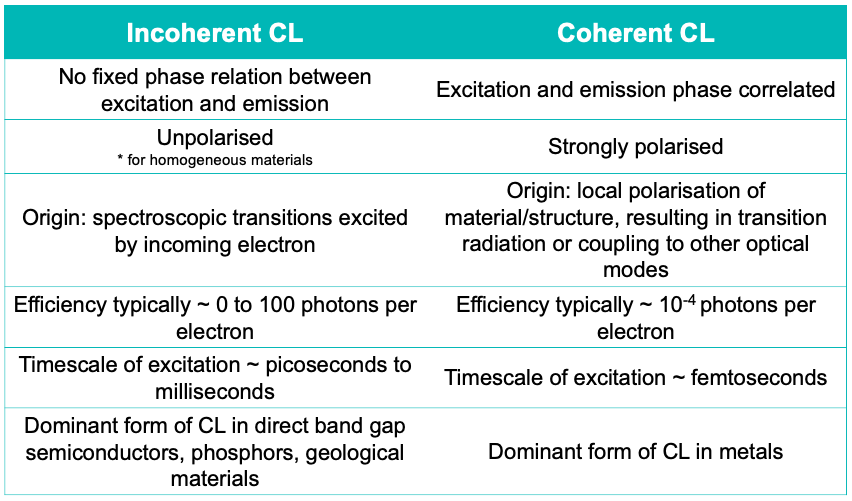
Incoherent cathodoluminescence emission occurs when the material is excited with an electron beam, where the primary electrons decelerate and deposit energy into the material.
An important property of incoherent CL is that there is no fixed phase relation between a photon that is emitted and an incoming excitation electron. This CL type is spontaneous, it’s not phase correlated with the excitation, and it is unpolarised if the medium is homogeneous. In geological materials, for instance, several defects may be present, some of which have a distinct CL signature. CL emission from these materials can be divided into extrinsic and intrinsic CL.
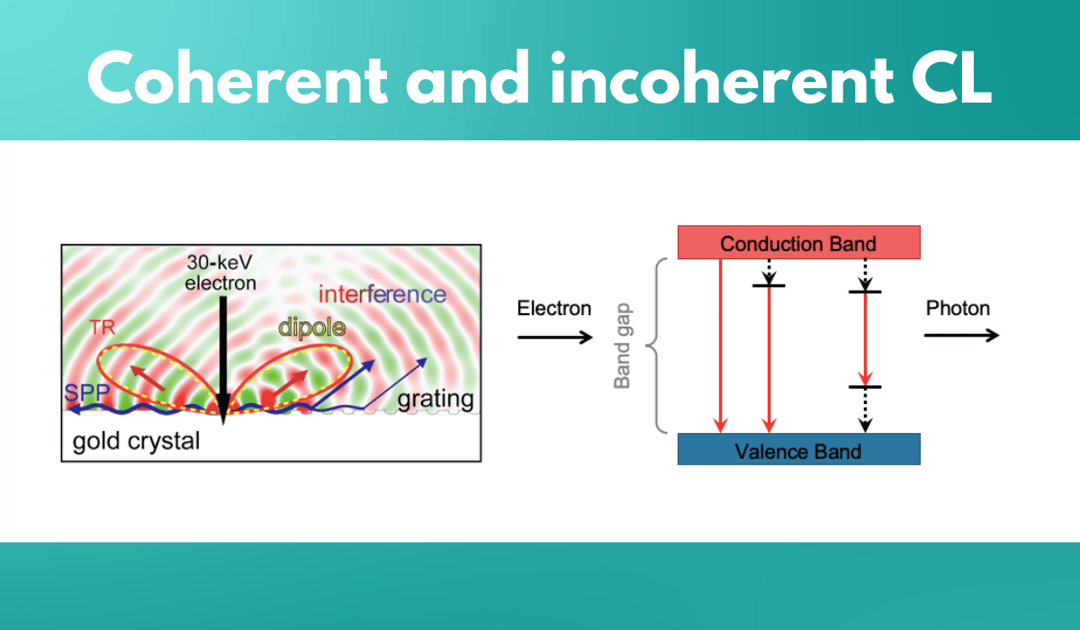
Coherent cathodoluminescence is generated whenever there is a difference in refractive index in the propagating path of the electron. If a material is in a vacuum, the interface between the material and the vacuum would allow the generation of coherent CL. For example, if a high energy beam is exciting a gold crystal, it polarises the material at the interface, and because of this, an oscillating charge is created. Measuring coherent CL essentially means probing the local density of optical states, and therefore, CL can be used to study optical phenomena at the nanoscale.
Coherent CL has two sources of radiation: transition radiation and surface plasmon polaritons. Unlike in incoherent CL, the emitted photon has a fixed phase relation with the incoming electron. In addition, the emitted light is always polarized. Coherent CL is always present but can be obscured by incoherent CL as it is a low-probability event. For example, it is present in materials such as direct bandgap semiconductors, phosphors, and ceramics, but it is obscured by the higher probability of incoherent CL emission. In metals and indirect bandgap semiconductors, on the other hand, incoherent CL is suppressed by non-radiative channels, because of which coherent CL emission dominates.
.png)