From the complex structures of viruses to extremely small forensic evidence, the revelations brought about by this technology have led to enormous developments in the scientific world. The wavelength of fast electrons is significantly smaller than that of visible light, creating images that were previously unobtainable with conventional light microscopy. For life scientists in particular, the main advantage of electron microscopy (from here on referred to as EM) is the contrast that the black-and-white high-resolution images reveal, providing essential information on the structure of a cell, organelle, or organic tissue.
EM does, however, come with a catch for life scientists. Imaging with an electron microscope requires processes such as freezing that immediately “kill” the sample in question. The lack of color also means that the scientist is not always entirely sure what they are observing. Instead, the user has to rely on intuition to identify certain parts. This is not to deny the value of the years of indispensable knowledge accumulated by the scientist. However, the integrity of research always relies on the absolute minimization of bias.
The disadvantages of such excellent technology does not mean that that researcher has to compromise on high accuracy. Developments in recent decades have allowed for the possibility to unite two types of microscopes into one system, bringing double the benefits. Fluorescence microscopy (FM), alone already an extremely valuable tool in life science research, can be combined with EM to form correlative light and electron microscopy, or CLEM. FM is particular form of light microscopy in which fluorescent light is used to image a sample. Samples are prepared to emit fluorescent light, either by staining, immunofluorescence, or with the use of fluorescent proteins. In CLEM, the user thus obtains a high-resolution black-and-white image with colors that serve as markings for particular parts of the sample.
Traditionally, the two methods were combined with separate instruments, in which FM would be used before turning to an electron microscope. The issue of bias, however, still comes into play, as the user nevertheless has to use a certain degree of intuition to manually overlay the images. This is why a more effective method is an integrated system that combines the two methods into one single instrument.
Using CLEM, the scientist can thus overcome several limitations brought about from using EM alone. Certain organelles or proteins in a sample can be readily identified. The labelling of parts is done in an objective manner with FM, thus enabling the scientist to more effectively validate his or her findings. FM also offers the chance to observe a still-living sample before the deadly EM snapshot, allowing one to capture specific “rare events” as they are happening at a high resolution.
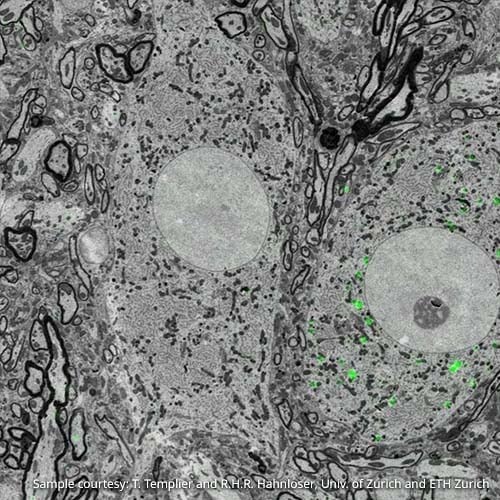
Figure 1: Overlay of fluorescence and electron images. Imaging was performed using the SECOM platform (DELMIC) mounted on a Quanta 250 FEG SEM (FEI). Sample courtesy: T. Templier & R.H.R. Hahnloser, University of Zürich, ETH Zürich
CLEM is highly beneficial for a range of research topics in the life sciences. In neurology, for example, a scientist can study neurons at a larger scale to identify their connectivity. Neurons are large cells that stretch over relatively “long” distances, originating in one part of the brain and perhaps ending in another. A researcher using CLEM can benefit from FM to trace a whole neuron, while employing EM to study more closely its structural properties [2]. (Figure 1). In the study of cell membranes, a researcher may be particularly interested in examining what processes are occurring at the membrane in time and space. For this, CLEM can be used to first identify the event of interest as it is happening using the less-invasive fluorescence microscope, and then make an image with EM for more detailed information on the context of these small events (Figure 2).
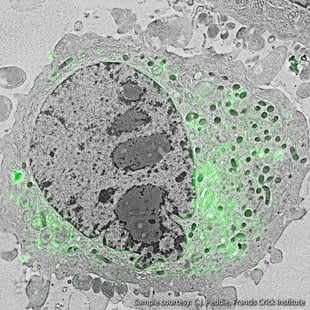
Figure 2: Localization of the lipid diacylglycerol within cellular membranes of HeLa cell expressing GFP-C1. Sample courtesy C.J. Peddie Francis Crick Institute
This is not to say, however, that FM is completely uninvasive to the sample. Furthermore, in CLEM, there is the potential for the fluorescent elements to be damaged by the electron microscope. Particular sample preparation methods have to be vigilantly applied to cause minimal damage. With continuous innovations in microscopy, more effective methods are being used to ensure that the sample remains as unchanged as possible [1] (for more information, see DELMIC's white paper on sample preparation).
The world of microscopy is constantly moving towards more efficient methods to create images at increasingly more tiny scales. For instance, recent developments in FM having managed to overcome the diffraction barrier, meaning that even optical microscopy is coming closer to having the high resolution that EM has to offer. Using a single microscopic technique is nevertheless limiting. It is correlative methods that can bring together the best of both worlds and unveil the world’s tiniest mysteries.
If you would like to learn more, we invite you to download our article which outlines in detail the added value of fluorescence microscopy to EM data:

Shedding Light on Electron Microscopy
References
[1] Brama, Elisabeth, et al. "Standard fluorescent proteins as dual-modality probes for correlative experiments in an integrated light and electron microscope." Journal of Chemical Biology 8.4 (2015): 179-188.
[2] Voortman, Lenard M. "Integration Without Compromise." Microscopy Today 22.06 (2014): 30-35.
Further reading
Yuan, Haifeng, et al. "Degradation of Methylammonium Lead Iodide Perovskite Structures through Light and Electron Beam Driven Ion Migration." The Journal of Physical Chemistry Letters 7 (2016): 561-566.
de Boer, Pascal, Jacob P. Hoogenboom, and Ben NG Giepmans. "Correlated light and electron microscopy: ultrastructure lights up!" Nature methods12.6 (2015): 503-513.
Brama, Elisabeth, et al. "Standard fluorescent proteins as dual-modality probes for correlative experiments in an integrated light and electron microscope." Journal of Chemical Biology 8.4 (2015): 179-188.
Voorneveld, Philip W., et al. "Loss of SMAD4 alters BMP signaling to promote colorectal cancer cell metastasis via activation of Rho and ROCK."Gastroenterology 147.1 (2014): 196-208.
Liv, Nalan, et al. "Scanning electron microscopy of individual nanoparticle bio-markers in liquid." Ultramicroscopy 143 (2014): 93-99.
Peddie, Christopher J., et al. "Integrated light and scanning electron microscopy of GFP-expressing cells." Methods in cell biology 124 (2014): 363-389.
Peddie, Christopher J., et al. "Correlative and integrated light and electron microscopy of in-resin GFP fluorescence, used to localise diacylglycerol in mammalian cells." Ultramicroscopy 143 (2014): 3-14.
Voortman, Lenard M. "Integration Without Compromise." Microscopy Today 22.06 (2014): 30-35.
Narváez, Angela C., et al. "Cathodoluminescence Microscopy of nanostructures on glass substrates." Optics Express 21.24 (2013): 29968-29978.
Liv, Nalan, et al. "Simultaneous correlative scanning electron and high-NA fluorescence microscopy." PLoS One 8.2 (2013): e55707.
Zonnevylle, A. C., et al. "Integration of a high-NA light microscope in a scanning electron microscope." Journal of microscopy 252.1 (2013): 58-70.
.png)