However, for cryo-ET to be effective, the sample must be thinner than the inelastic mean-free-path length of electrons, which is roughly 350 nm for 300 keV electrons [4]. Thicker samples degrade image quality due to an increase in inelastically scattered electrons.
Challenges in sample preparation
Most biological samples, such as eukaryotic cells or tissues, are too thick for cryo-ET and require thinning. Cryo-ultramicrotomy is one method used to reduce sample thickness by cutting the sample into ribbons of vitreous sections that are thin enough for imaging [5]. However, this technique is challenging and can introduce artifacts such as knife marks, compression, or crevasses [6].An alternative approach involves using a focused ion beam under cryogenic conditions (cryo-FIB) to mill the samples [7,8]. This technique is effective in preparing samples for cryo-ET without the artifacts associated with cryo-ultramicrotomy [9,10].
Despite the advantages, the cryo-FIB milling process involves multiple handling, transfer, milling, and imaging steps, which can be delicate and risky. The thin, fragile lamellae are easily damaged, and the constant need for cooling to prevent devitrification makes the samples susceptible to contamination. Ice contamination, particularly from ice crystals, can obscure features in the lamellae and even render them unusable for data acquisition.
Possible artefacts arising during handling, transfer and processing
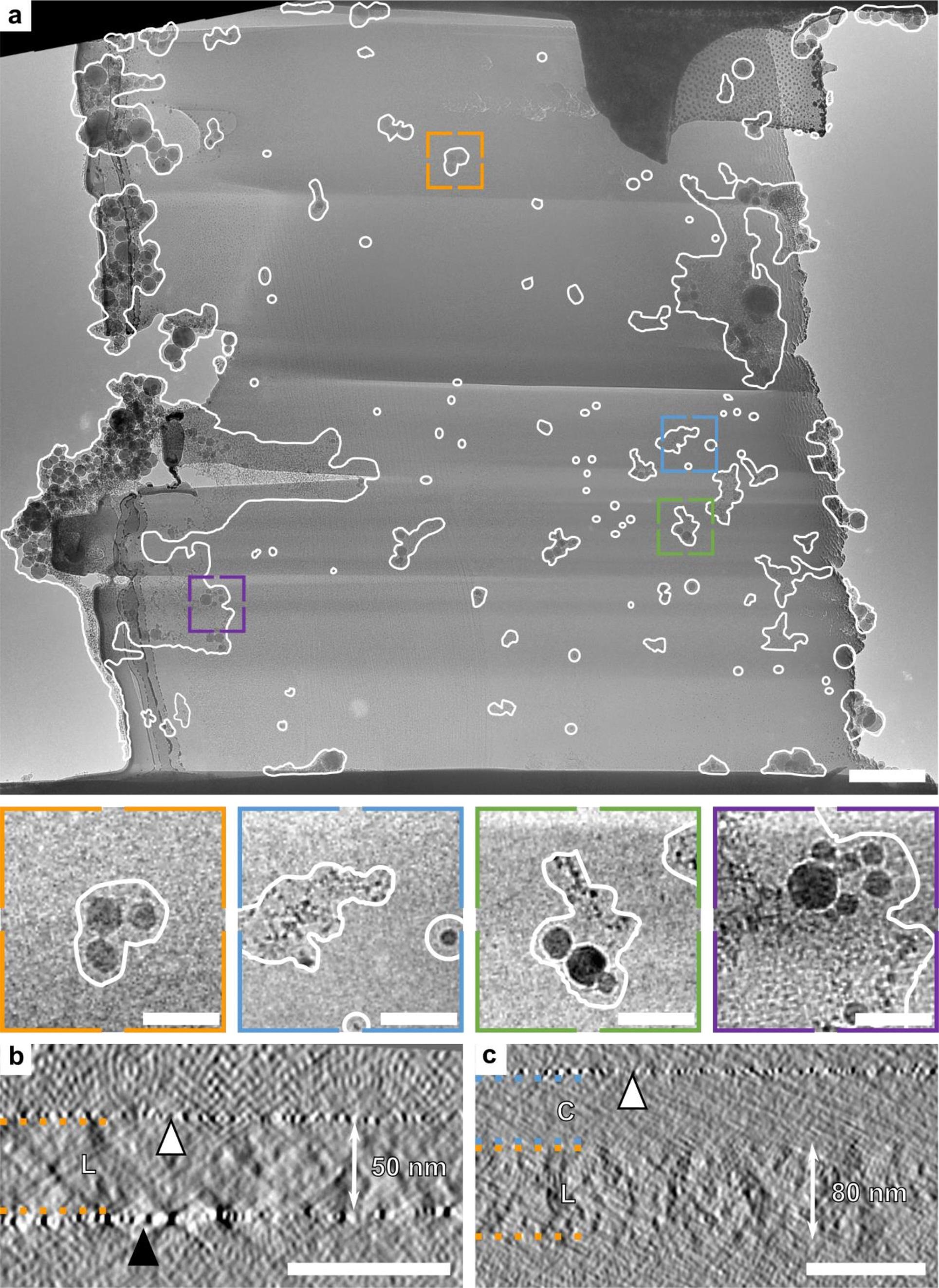
a) A standard-prepared lamella, without hardware upgrades, shows ice crystal contamination of up to 20% when transferred in a moist environment. Scale bar: 250 nm for close-ups, 1 µm for the overview.
b,c) Tomogram side views of two lamellae from the same milling session. The lamella (L) in (b), outlined in orange, was polished last and immediately removed from the SEM, showing no water deposition. The sputter layer is marked with a white triangle, and platinum redeposition is indicated by a black triangle. The lamella (L) in (c), also outlined in orange, was polished earlier and left in the SEM, resulting in a 50 nm water deposition layer (C), outlined in blue, atop the 80 nm lamella, reducing its quality. The platinum coating is marked with a white triangle. Scale bars: 100 nm
Recent advancements include the introduction of software packages that automate the milling process, allowing for batch milling of multiple lamellae in a 24-hour period. However, the extended time samples spend in the microscope during automation increases the risk of contamination and limits the number of high-quality lamellae produced per session.
To optimize lamella production for cryo-ET and fully leverage the benefits of automated cryo-FIB milling, several key issues must be addressed:
- Reducing frost contamination during sample handling and transfer.
- Avoiding amorphous ice contamination inside the cryo-FIB/scanning electron microscope (SEM).
- Streamlining the handling of the thin and fragile lamellae to avoid cracking.
Integrated workflow for reduced contamination
The study introduced several measures to prevent frost contamination and amorphous ice in the vacuum system:- A glove box was used to create a controlled environment for sample handling.
- A high vacuum cryo transfer system was employed for safe sample transfer.
- A cryo-shield was implemented to protect the samples from ice formation.
- A cryo-shutter was used to maintain the vacuum integrity during sample exchange.
These measures collectively ensured a nearly water-free environment, which is crucial for cryo-FIB milling and other sensitive procedures. Additionally, a new preparation station was introduced to streamline the handling of fragile lamellae, aiming to avoid sample handling artifacts with maximal user convenience.
Schematic of the glove box, the preparation station, and the high vacuum cryo transfer system

a) The glove box provides a dry environment for sample handling, with nitrogen purging and a cold trap to minimize humidity. LN2 is accessible for in-box use, and heated tool holders are installed for convenience. A load lock allows tool insertion without disrupting the humidity barrier. Scale bar: 600 mm.
b) The new preparation station offers a flexible and secure setup for sample handling. It includes a liquid nitrogen bath, interchangeable preparation modules, and adapters for various transfer systems. The station is designed for both left- and right-handed users and can be rearranged according to the operator's preference. It is mounted on a rail system for easy movement within the glove box. Scale bar: 400 mm.
c) The high-vacuum cryo-transfer system features a transfer rod, vacuum chamber, LN2 reservoir, valve, and adapters for the cryo-FIB/SEM and glove box. Samples are picked up by the transfer rod and placed in the chamber, where they are cooled by the LN2 reservoir. The LN2 also functions as a cryo-pump to maintain vacuum levels during disconnection from the pumps. The LN2 supply lasts about 30 minutes. After the shuttle is parked, valves are adjusted to prepare for sample transfer to the cryo-FIB/SEM. Scale bar: 300 mm.
The improved and new hardware components successfully reduced contamination, enabling an almost 24/7 operation and preventing limited lamella production per session. The introduction of the AutoTEM Cryo software application allowed for supervised automated batch milling of many lamellae, reducing the time spent on the microscope and enabling the preparation of up to 27 lamellae per session. The increased throughput also facilitated quality assessment routines and exploration of different milling parameters, such as the influence of high ion currents on the quality of the cryo-lamellae.
Glove Box, sample preparation, and transfer
The study was conducted using a glove box that was purged with dry nitrogen to maintain a low-moisture environment. Before introducing the sample, the liquid nitrogen reservoir and the preparation station were filled with liquid nitrogen to keep the sample at cryogenic temperatures. The EM grids were then loaded into the cryo-FIB shuttle and transferred to the cryo-FIB/SEM instrument via a high-vacuum cryo-transfer system.
During the milling process, the glove box and preparation station were heated to 40-50°C to prevent frost contamination. After milling, the sample was transferred back to the glove box, and the autoloader grids were unloaded, rotated by 90°, and mounted into an autoloader cassette. The cassette was then loaded into the NanoCab and transferred to the electron cryo-microscope for further analysis.
Hardware modified Cryo-FIB/SEM
Cryo-shield and cryo-shutter

a) Schematic of the cryo-FIB/SEM chamber, showing the cryo-shield (SH) next to the electron column (EC) and ion column (IC). The cryo-shield has a surface area of approximately 880 cm². With a pump strength of about 15 l cm⁻² s⁻¹ [11], the total pumping speed is estimated at 13,000 l s⁻¹. A cryo-shutter (CS) can be inserted during milling to further reduce the partial pressure near the sample. The shuttle, with autoloader grids (white triangle), is positioned beneath the cryo-shutter via the cryo stage (ST). Two cryo-shutter types were tested, with Type II offering better contamination reduction by covering most of the grid. Scale bar: 25 mm.
b) Camera image from the chamber's rear, showing the cryo-shutter almost completely covering the autoloader grids. The white triangle indicates the stage heater's position (not installed in this image). Scale bar: 25 mm.
c) Ion image of the cryo-shutter Type II, showing the EM grid through the shutter's hole. The hole's diameter is approximately 1 mm to ensure full grid protection. For electron imaging, the shutter is automatically retracted. These enhancements allow the sample to be prepared and stored without amorphous ice contamination. Scale bar: 300 µm.
The cryo-FIB/SEM instrument used in the study was equipped with a larger cryo-shield, a cryo-shutter, and a stage heater, all of which were custom-made modifications. The instrument was also equipped with a gas injection system (GIS) and a platinum sputter coater. After insertion, the grids were coated with a protective organometallic platinum layer to prevent damage during milling.
The scanning electron imaging was performed at 2-5 kV acceleration voltage and a maximum electron current of 28 pA, while the ion optics were operated at 30 kV acceleration voltage with various ion currents (50-700 pA). The alignment of the ion optics was crucial and was performed weekly to avoid image shifts.
Quantification of frost and amorphous ice contamination
To quantify the reduction of frost contamination, three lamellae preparations were performed with the standard configuration of the cryo-FIB/SEM, and three preparations with the developed hardware upgrades (glove box, preparation station, and high vacuum cryo transfer). After milling, overview images of each lamella were analyzed for frost contamination, and the ratio of the contaminated area to the total lamella area was calculated. The experiments with the hardware upgrades were performed with a room humidity of 50-60%, while the standard configuration experiments were performed with a room humidity of approximately 30%.Additionally, the growth of amorphous ice contamination was measured by taking baseline images one hour after cooling the cryo-FIB/SEM and then taking subsequent images after at least two hours. Imaging conditions were specified, and measurements were taken at multiple positions on the grid.
Results and conclusion
The authors conducted a quality assessment of the automated lamellae preparation and demonstrated that the streamlined workflow not only increases the overall throughput of the lamellae production but also generates results suitable for in-situ high-resolution investigations. The success rates for rough milling and polishing were quantified, with the overall efficiency of the total workflow estimated to be around 80%, making the process more efficient and reducing operator time significantly.
To summarize, the study illustrated that the introduction of these improvements not only increases the overall throughput of lamellae production but also generates results suitable for in-situ high-resolution investigations.
Based on the study - https://www.sciencedirect.com/science/article/pii/S1047847721000484
References
- Bartesaghi, A., Lecumberry, F., Sapiro, G., & Subramaniam, S. (2012). Protein secondary structure determination by constrained Single-Particle Cryo-Electron tomography. Structure, 20(12), 2003–2013. https://doi.org/10.1016/j.str.2012.10.016
- Grange, M., Vasishtan, D., & Grünewald, K. (2017). Cellular electron cryo tomography and in situ sub-volume averaging reveal the context of microtubule-based processes. Journal of Structural Biology, 197(2), 181–190. https://doi.org/10.1016/j.jsb.2016.06.024
- Schur, F. K. M., Obr, M., Hagen, W. J. H., Wan, W., Jakobi, A. J., Kirkpatrick, J. M., Sachse, C., Kräusslich, H., & Briggs, J. a. G. (2016). An atomic model of HIV-1 capsid-SP1 reveals structures regulating assembly and maturation. Science, 353(6298), 506–508. https://doi.org/10.1126/science.aaf9620
- Vulović, M., Ravelli, R. B., Van Vliet, L. J., Koster, A. J., Lazić, I., Lücken, U., Rullgård, H., Öktem, O., & Rieger, B. (2013). Image formation modeling in cryo-electron microscopy. Journal of Structural Biology, 183(1), 19–32. https://doi.org/10.1016/j.jsb.2013.05.008
- Al-Amoudi, A., Norlen, L. P., & Dubochet, J. (2004). Cryo-electron microscopy of vitreous sections of native biological cells and tissues. Journal of Structural Biology, 148(1), 131–135. https://doi.org/10.1016/j.jsb.2004.03.010
- Alamoudi, A., Studer, D., & Dubochet, J. (2005). Cutting artefacts and cutting process in vitreous sections for cryo-electron microscopy. Journal of Structural Biology, 150(1), 109–121. https://doi.org/10.1016/j.jsb.2005.01.003
- Marko, M., Hsieh, C., Schalek, R., Frank, J., & Mannella, C. (2007). Focused-ion-beam thinning of frozen-hydrated biological specimens for cryo-electron microscopy. Nature Methods, 4(3), 215–217. https://doi.org/10.1038/nmeth1014
- Rigort, A., Bäuerlein, F. J. B., Leis, A., Gruska, M., Hoffmann, C., Laugks, T., Böhm, U., Eibauer, M., Gnaegi, H., Baumeister, W., & Plitzko, J. M. (2010). Micromachining tools and correlative approaches for cellular cryo-electron tomography. Journal of Structural Biology, 172(2), 169–179. https://doi.org/10.1016/j.jsb.2010.02.011
- Hagen, C., Dent, K. C., Zeev-Ben-Mordehai, T., Grange, M., Bosse, J. B., Whittle, C., Klupp, B. G., Siebert, C. A., Vasishtan, D., Bäuerlein, F. J., Cheleski, J., Werner, S., Guttmann, P., Rehbein, S., Henzler, K., Demmerle, J., Adler, B., Koszinowski, U., Schermelleh, L., . . . Grünewald, K. (2015). Structural basis of vesicle formation at the inner nuclear membrane. Cell, 163(7), 1692–1701. https://doi.org/10.1016/j.cell.2015.11.029
- Mahamid, J., Pfeffer, S., Schaffer, M., Villa, E., Danev, R., Cuellar, L. K., Förster, F., Hyman, A. A., Plitzko, J. M., & Baumeister, W. (2016). Visualizing the molecular sociology at the HeLa cell nuclear periphery. Science, 351(6276), 969–972. https://doi.org/10.1126/science.aad8857
- Bello, I. (2017). Vacuum and ultravacuum. In CRC Press eBooks. https://doi.org/10.1201/9781315155364
.png)







