Drug discovery plays a crucial role in healthcare, but the process is very costly and time-consuming. Fortunately, advancements in molecular and structural biology can accelerate this process by providing a better and more detailed understanding of the drug targets [1].
Cryo-electron microscopy (cryo-EM) is now widely used to obtain high-resolution reconstructions of macromolecular structures, revealing the molecular basis of diseases, and thereby facilitating the design of novel drugs. The workflow of single particle analysis (SPA), a subtype of cryo-EM, includes sample preparation, cryo-EM grids setup, imaging, data collection and pre-processing, map reconstruction, model building, and lastly, structural analysis of the protein (Figure 1) [1].
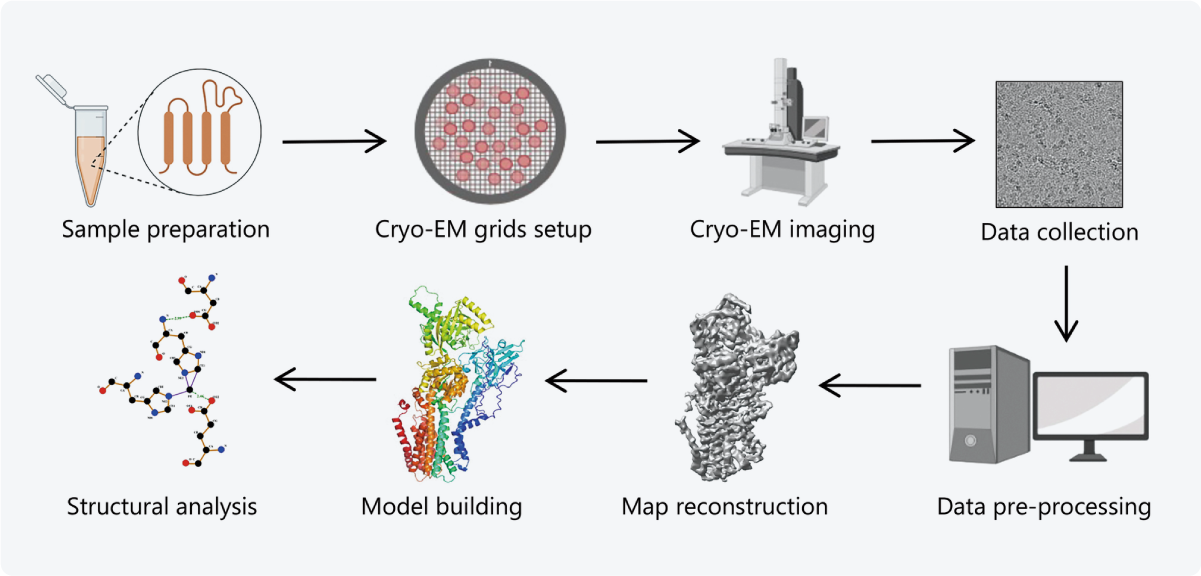 Figure 1 The workflow of single particle analysis, a subtype of cryo-EM. Taken from [1].
Figure 1 The workflow of single particle analysis, a subtype of cryo-EM. Taken from [1].
Unveiling the molecular basis of diseases
In the field of structural biology, cryo-EM thus emerges as a powerful tool for studying macromolecules. It offers several advantages over related techniques such as X-ray crystallography and NMR, primarily because it doesn’t require large sample sizes or complex crystallization processes and it allows for a wider variety of proteins to be imaged, such as membrane proteins [2].
Moreover, cryo-EM enables the observation of the macromolecular structures from their native physiological environment at near-atomic resolution. It is widely used for reconstructing large protein structures, and due to recent advancements, its applicability is extended to small proteins as well [1]. Cryo-EM is now leading to novel discoveries in the molecular pathology of many diseases, such as Parkinson’s disease, HIV, and more [3][4].
Cryo-EM is also at the forefront of the discovery of novel drug targets for SARS-CoV-2. First of all, it has revealed the spike protein in different conformational states, enabling an understanding of the dynamics of the protein when it interacts with the host [5][6]. Understanding these interactions is key to the development of vaccines [7]. Secondly, cryo-EM has characterized the complexes that the spike protein forms with receptors and antibodies. This has led to the identification of antibodies for therapeutic use (Figure 2) [8][9][10].
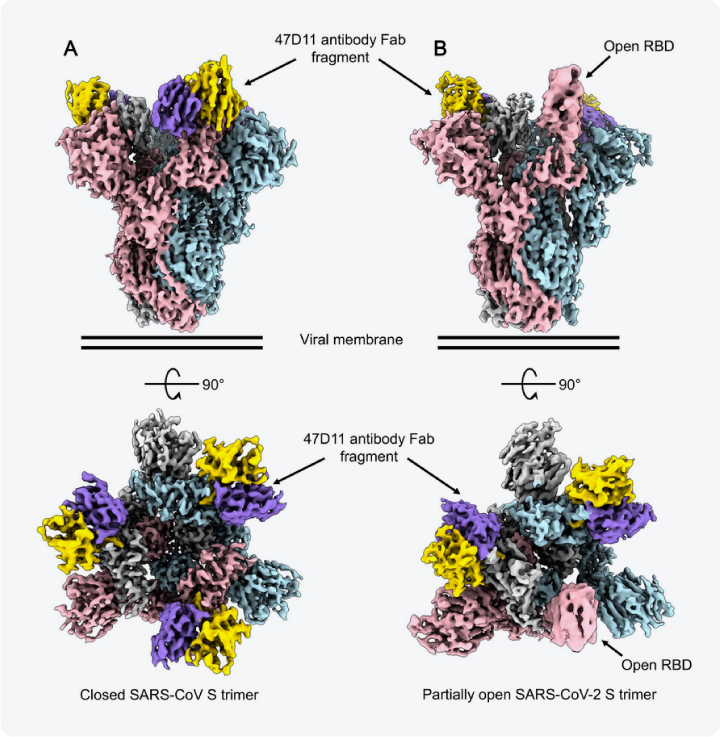 Figure 2 Reconstructions of the SARS-CoV-2 spike protein bound to 47D11 antibody Fab fragments. The spike protomers are colored pink, blue, and gray, and the 47D11 antibody is colored purple and yellow. The protein can be seen to have a closed conformation when three antibodies are bound, and a partially open conformation when only two of the antibodies are bound. Taken from [9].
Figure 2 Reconstructions of the SARS-CoV-2 spike protein bound to 47D11 antibody Fab fragments. The spike protomers are colored pink, blue, and gray, and the 47D11 antibody is colored purple and yellow. The protein can be seen to have a closed conformation when three antibodies are bound, and a partially open conformation when only two of the antibodies are bound. Taken from [9].
Screening novel drugs
Apart from its use in reconstructing macromolecular structures, cryo-EM also holds great potential in directly aiding the screening of novel drugs. It could be used to visualize and study the molecular interactions between drugs and proteins directly isolated from their native environment.
Additionally, cryo-EM enables researchers to study heterogeneity in the sample so that multiple conformations of a protein can be distinguished from one another. This wealth of data could enhance our understanding of the interactions between the drugs and their targets and make it possible to optimize the drug's targeting ability.
Ongoing improvements
Despite its potential, cryo-EM has not been widely adopted in the field of drug development, mainly due to its challenges in sample preparation. Issues such as protein aggregation, destruction, insufficient protein amounts, and ice contamination often cause many grids to be unusable. As a result, cryo-EM suffers from labor-intensive processes and low throughput [11].
However, a combination of sample preparation improvements, such as protection against ice contamination, and rapid identification of suitable samples, together with improved image processing algorithms, could make cryo-EM a more attractive tool for drug development [4].
Cryo-EM has thus enabled significant advancements in understanding the molecular basis of diseases, including Parkinson's disease, HIV, and SARS-CoV-2. It has provided insights into the dynamics of protein interactions, which are crucial for vaccine development and the identification of therapeutic antibodies. Moreover, cryo-EM holds promise in directly aiding the screening of novel drugs. Improvements in sample preparation techniques and image processing algorithms have the potential to make cryo-EM capable of revolutionizing the drug development process.
References
[1] Zhu, K. F. et al., Military Med Res 10, 10 (2023)
[2] García-Nafría, J. et al., Annual Review of Pharmacology and Toxicology, 60:1, 51-71 (2020)
[3] Benjin, X. et al., Protein Science. 29, 872–882 (2020)
[4] Fernandez-Leiro, R. et al., Nature 537, 339–346 (2016)
[5] Zhou, T. et al., Cell Host & Microbe 28, 6, 867-879 (2020)
[6] Wrapp, D. et al., Science 367,1260-1263 (2020)
[7] Rapp, M et al., Trends Biochem Sci., 47(2), 117-123 (2022)
[8] Cerutti, G. et al., Structure., 29(7), 655-663 (2021)
[9] Fedry, J. et al., Sci Adv., 7(23), (2021)
[10] Wang, Q. et al., Cell 181, 4, 894-904 (2020)
[11] Ceska, T. et al., Biochem Soc Trans, 47 (1), 281–293 (2019)
.png)