The efficiency of perovskite solar cells is increasing further and further, and a record of 25.7% power conversion efficiency (PCE) has now been measured [1]. These highly efficient solar cells can provide a much needed impetus to further spur the growth of solar energy, and open up new application areas. However, most perovskite solar cells contain lead (Pb), which is toxic even in small doses [2].
Although proper encapsulation and end-of-life recycling schemes minimize the risk of lead leaking into the environment, researchers are looking for alternative compositions that eliminate the need for lead altogether [3]. However, lead-based perovskites are still the undisputed leaders when it comes to efficiency and stability. Is there a realistic alternative? To answer that question, we have to zoom in to the atomic scale of perovskites.
Metal halide Perovskite solar cells
The term perovskite originally refers to the mineral calcium titanate (CaTiO3) but it has come to be used as a broad term describing any material that adopts the same crystal structure (Figure 1). A specific instance of this is the metal halide perovskites (ABX)3, in which B is a divalent metal ion, most commonly Pb, and X is a halide ion (Cl, Br, I or a mixture). The A-site is typically occupied by caesium or a small organic molecule such as methylammonium (MA) or formamidinium (FA).
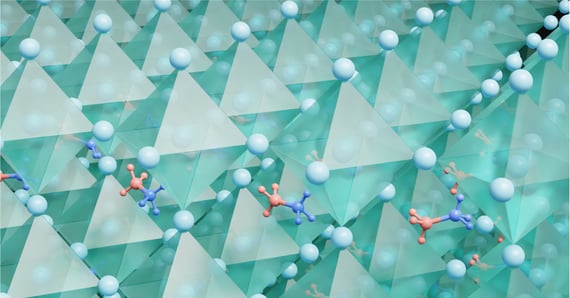 Figure 1: Rendering of the perovskite crystal structure. Small organic molecules (MA) can be seen to occupy the voids in a network of inorganic octahedra (BX6).
Figure 1: Rendering of the perovskite crystal structure. Small organic molecules (MA) can be seen to occupy the voids in a network of inorganic octahedra (BX6).
One of the aspects that makes metal halide perovskites such attractive materials for solar cells is the fact that they are direct band gap materials, yet they have long lifetimes of generated charge carriers. This combination is difficult to achieve with current semiconductors used in solar cells [4]. Add to this the fact that perovskites do not require complex and high-temperature deposition processes, and it becomes clear why interest in these materials as an active layer for solar cells is surging. Could the same be achieved when substituting lead?
A logical first choice when considering alternatives to lead is substitution by elements within the same group of the periodic table.
The major contenders from this perspective are tin (Sn) and germanium (Ge) owing to their similar electronic configuration and suitable ionic radii (Figure 2). While fundamentally both these materials possess suitable optoelectronic properties, their efficiency is lagging behind.
Both Sn and Ge based perovskites are chemically unstable in their +2 oxidation state, and as a result readily oxidize when exposed to ambient conditions. While germanium can be largely discarded as a feasible alternative to lead owing to its high cost and poor stability [5], the use of tin in perovskites holds promise if oxidation can be prevented [6]. Research efforts aimed at improving the crystallization, adding reducing agents and encapsulation are all contributing to this end. It remains to be seen if ultimately tin could become a viable alternative to lead in perovskite solar cells.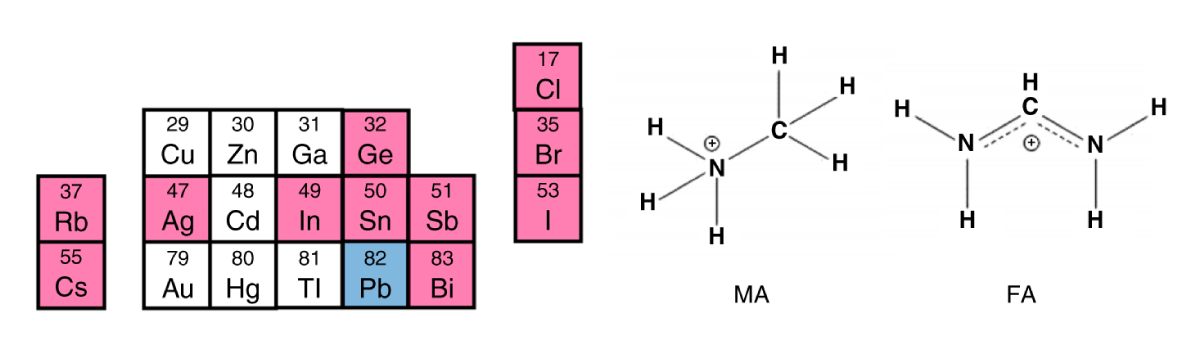 Figure 2: Part of the periodic table highlighting some of the potential elements considered for lead-free perovskites, including A site cations (Rb, Cs), metals and halides (Cl, Br, I) along with the molecular structure of methylammonium (MA) and formamidinium (FA). Taken from [3].
Figure 2: Part of the periodic table highlighting some of the potential elements considered for lead-free perovskites, including A site cations (Rb, Cs), metals and halides (Cl, Br, I) along with the molecular structure of methylammonium (MA) and formamidinium (FA). Taken from [3].
Another approach that is explored to substitute lead is the concept of double perovskites.
In these compositions, the divalent lead ions are replaced by a monovalent and a trivalent cation belonging to different groups of the periodic table. The prime example of this being CsAgBiBr6, in which lead is replaced by silver and bismuth. Although the stability of double perovskites tends to be high, they also typically have band gaps that are larger than the ideal band gap for solar cells (Figure 3). This ultimately limits the maximum attainable efficiency, as part of the incoming solar spectrum cannot be absorbed.
A recent report by a team of researchers from the Beijing University of Technology, Nankai University and the Beijing Computational Science Research Center found that the band gap of these materials can be lowered through hydrogenation to better match the solar spectrum [7]. Replacing bismuth by thallium (Tl) can also reduce the band gap of double perovskites substantially, although the use of this highly toxic element defeats the purpose of lead substitution [8]. Despite these limitations, wide band gap double perovskites like CsAgBiBr6 might instead find use as top cells in tandem solar cells or as photodetectors or light sources in the visible range of the electromagnetic spectrum.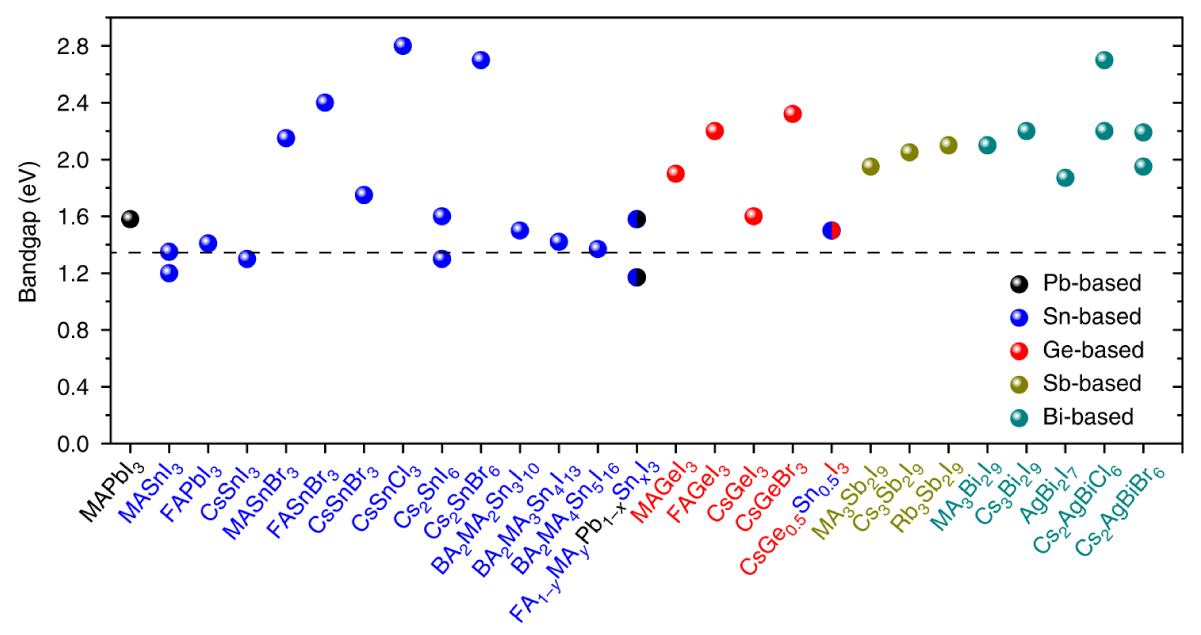 Figure 3: The band gap of perovskites based on Pb, and based on alternative elements. The optimal bandgap for single-junction solar cells, indicated by the dashed line, is 1.34 eV. Adjusted from [3].
Figure 3: The band gap of perovskites based on Pb, and based on alternative elements. The optimal bandgap for single-junction solar cells, indicated by the dashed line, is 1.34 eV. Adjusted from [3].
Finally, low-dimensional and vacancy-ordered perovskites structures provide an even larger space of possible lead-free compositions to explore.
Complete substitution of lead by bismuth or antimony (Sb) for instance gives rise to vacancy-ordered perovskites such as Cs3Sb2I9 and Cs3Bi2I9. Ghimire et al. [9] recently synthesized Cs3Bi2I9 perovskite nanosheets and used cathodoluminescence spectroscopy to study the optical properties of a single isolated nanosheet.
The authors observed CL that is consistent with strongly bound and self-trapped excitons, suggesting that excited charge carriers are confined to the isolated octahedra in these structures. Such strong confinement limits the mobility of charge carriers in the active layer, and complicates extraction of charges in a solar cell. As with the double perovskites, many of the vacancy-ordered perovskites are indirect wide band gap materials.
Overall, while these Perovskites have shown to be stable and capable of light-harvesting, their applicability in solar cells is expected to be minimal for the above reasons. Demonstrated solar cell efficiencies have thus far indeed been well below 1% for these materials [10].
To conclude, while perovskite solar cells are breaking record after record in terms of efficiency, concerns regarding the use of lead has been a driving force behind the exploration of lead-free alternatives. Many different compositions have been proposed, some of which have been synthesized and characterized, while others remain merely hypothetical.
Delmic recently joined forces with other academic and industrial parties in the PerFoRM consortium, which aims to develop sustainable and scalable fabrication technologies for lead-free perovskites. Although it remains an open question whether any of these materials can match the performance of lead-based perovskite solar cells, it is beyond doubt that material characterization will continue to play an important role in the search for lead-free alternatives.
References
[1] NREL Best Research-Cell Efficiency Chart, 2022
[2] Exposure to Lead: a Major Public Health Concern. Geneva: World Health Organization; 2010.
[3] Weijun Ke, Mercouri G. Kanatzidis, Nat. Commun. 10, 965 (2019)
[4] Thomas M. Brenner et al., Nat. Rev. Mater. 1, 15007 (2016)
[5] Giorgio Schileo, Giulia Grancini, J. Mater. Chem. C, 9, 67-76 (2021)
[6] Shuyan Shao et al., Adv. Energy Mater., 8, 1702019 (2018)
[7] Zeyu Zhang et al., Nat. Commun. 13, 3397 (2022)
[8] Adam H. Slavney et al., Angew. Chem. Int. Ed., 57, 12765 (2018)
[9] Sushant Ghimire et al., Nanoscale, 15, 2096-2105 (2023)
[10] Feliciano Giustino, Henry J. Snaith, ACS Energy Lett., 1, 6, 1233–1240 (2016)
.png)