At the time, cathode rays were only known as the consequence of an electric current that was passed through a vacuum tube. It was observed that electrically charged particles would collide with atoms at the end of the tube and excite them, thus causing them to fluoresce, or emit fluorescent light. It was further made evident that these were rays travelling in a straight line from one end of the tube to the next, by placing a shape in the middle of the tube and observing that very shape casting a shadow at the end of the tube.
J.J. Thomson investigated further into the properties of these mysterious rays. The debate during this time was whether cathode rays are “immaterial” or in fact mark the path of charged particles. Thomson thus experimented with ways to manipulate the cathode rays. What he found was four things: (1) cathode rays could be magnetically deflected, (2) the cathode rays are themselves negatively charged, (3) cathode rays can be deflected by an electric field, and finally, (4) cathode rays consist of extremely small and highly charged particles. Cathode rays were thus found to be particles of matter and charges of negative electricity simultaneously. Thomson referred to these particles as corpuscles, and they later became known as electrons.
One might ask what this has to do with cathodoluminescence microscopy. Cathodoluminescence imaging is the process of analyzing the visible radiation that is generated by cathode rays, or what is now recognized to be a beam of electrons. Given its role in fundamental processes on a subatomic level, cathodoluminescence is a highly valuable tool in microscopy for analyzing the properties of materials at an extremely small scale. Incidentally, the practical use of cathode rays are more ubiquitous than one might expect: before OLED, LCD and plasma televisions entered the scene, TV images were displayed via the use of a cathode ray tube.
In SEM cathodoluminescence microscopy, cathodoluminescence is combined with a scanning electron microscope (SEM). In such a system, a tightly focused beam of electrons is fired at a material and the researcher examines the electrons that bounce back. The electron beam which the SEM fires at the sample also excites the material. As the material returns to a ground state, it emits photons, which are then analyzed with the cathodoluminescence system. In this way, one can observe the optical properties of a material at the same time that they can study the structural information provided by the SEM.
However, if one wants optical information, why not just use a conventional light microscope? Well, as discussed in our previous blog post, a common problem in optical microscopy is the diffraction barrier, in which objects that are approximately less than half the wavelength of the light used cannot be distinguished from one another. SEM and cathodoluminescence microscopists do not face this problem, as it is electrons that are being fired with extreme precision that operate as the source of optical information.
Also compared to a conventional scanning electron microscope, cathodoluminescence imaging has several advantages. The photons that are emitted as a response to the electron beam contribute a wealth of information on materials that a SEM alone cannot provide. For example, one can examine the electronic properties of nanophotonic systems and nanoscale optoelectronic devices. In this case, the light emitted from the sample provides information on the local density of states, or the states that can be occupied by a photon. In other words, one can analyze how well light couples to matter and vice versa, of course an essential question for nanophotonic and optoelectronic research.
One particularly interesting case that attests to the irreplaceable value of SEM cathodoluminescence is research on the optical response of metallic nanoparticles, about which a paper was recently published in Nano Letters. The operation of the metallic nanostructure in question depends in part on how it is excited by an external stimulus. This stimulus can be a beam of light, but such a beam cannot be focused on a spot that is small enough, thus limiting the ability to precisely control the optical behavior of the structure. Cathodoluminescence provided the solution to this problem, and the researchers were able to study what colors of light are efficiently emitted when it is excited by an electron beam at different positions.
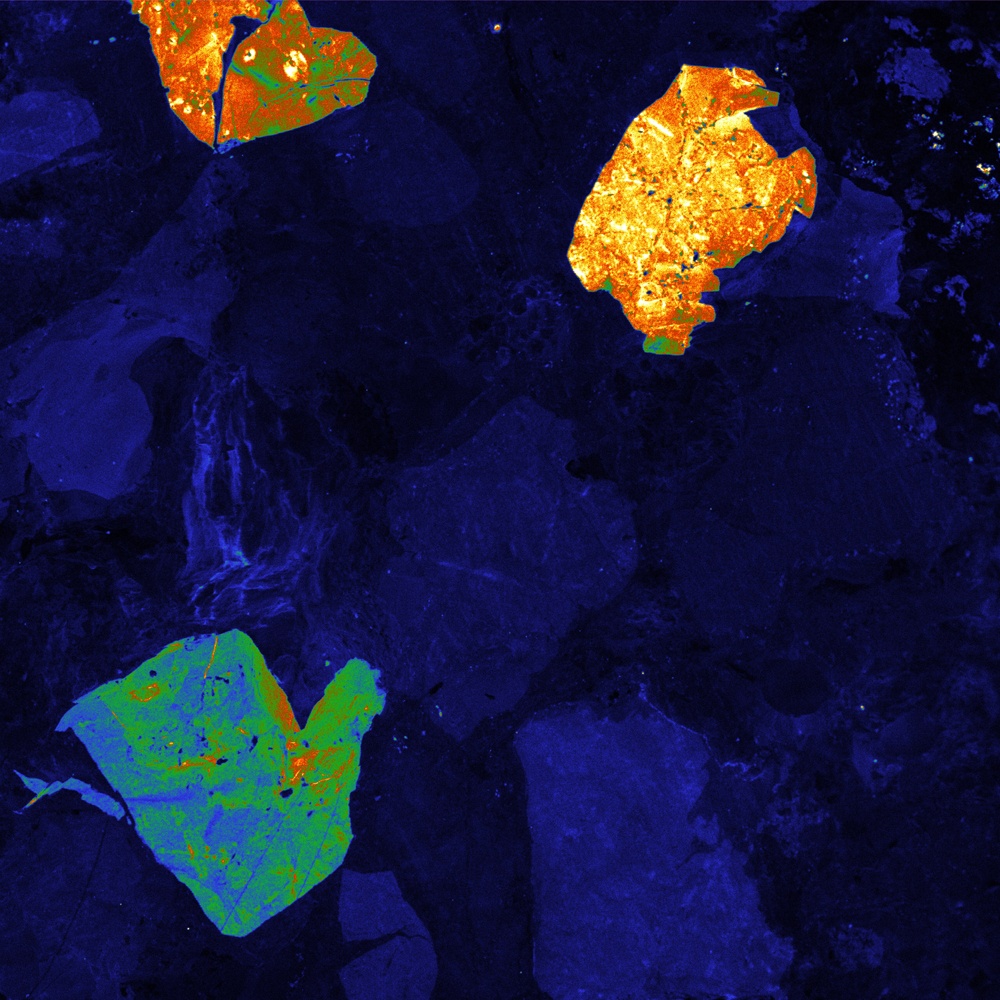
Cathodoluminescence also has several other applications. In the study of rocks and minerals, one can investigate the distribution of trace elements in a sample, or conduct provenance studies to examine the history of a geological sample. One particular example in which cathodoluminescence is especially advantageous is the study of quartz in sandstone. In combination with a scanning electron microscope, cathodoluminescence can very closely and intricately map the composition of quartz in a sample in order to uncover the geological history and the porosity of the rock (to learn more, download our white paper: Cathodoluminescence Imaging on Sedimentary Rocks: Quartz Sandstones). This subject of research is particularly of relevance to the fossil fuel industry. Clearly, cathodoluminescence imaging is a versatile tool for a wide range of research in the study of materials and optics.
In the advent of the incidental discovery of the electron, electrons have been utilized for a wide range of microscopy techniques due to their inherent properties and extremely small size. It is a curious fact that cathodoluminescence played such an important role in first revealing the electron to science. This, however, attests to the very relevance of cathodoluminescence imaging as a technique for studying materials at the nanoscale.
If you want to learn more, see our list of cathodoluminescence resources.
DELMIC produces a SEM cathodoluminescence system, the SPARC for use in materials science, optics, nanophotonics, and geosciences. You can download the brochure below:
.png)



 Download SPARC brochure
Download SPARC brochure

