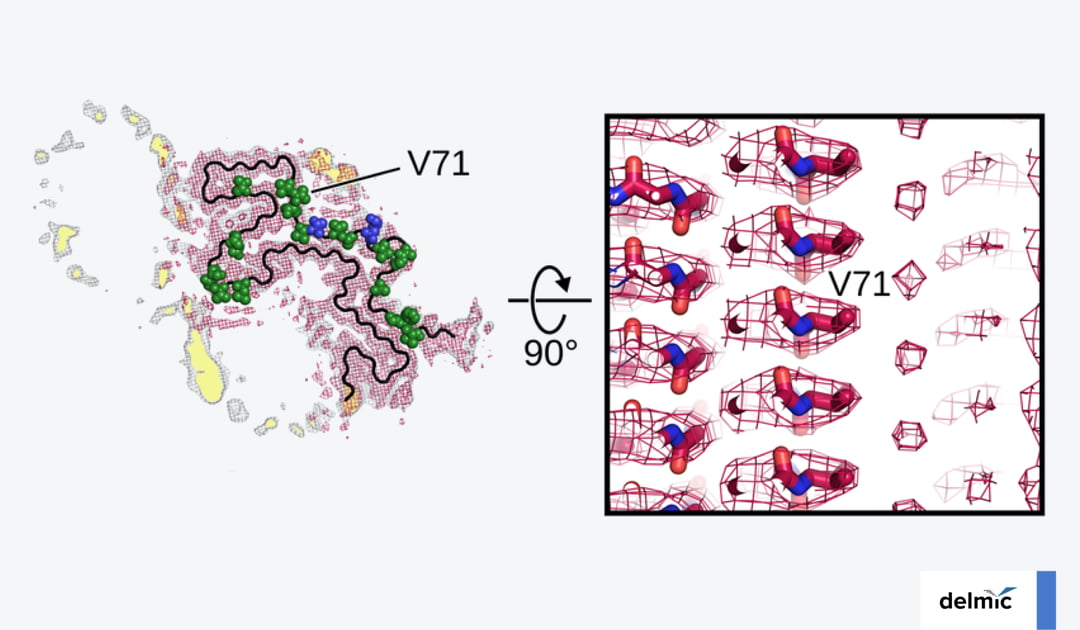
Parkinson's disease (PD) is a neurological disorder that affected over 8.5 million individuals in 2019, and death due to PD is fast growing [1]. Despite its early discovery in 1817 and over a century of research, the exact cause of PD is still unclear. But advances in cryo-electron microscopy (cryo-EM) now allow researchers to zoom in on the atomic level to understand the molecular biology of PD.
In PD, nerve cells in the substantia nigra pars compacta break down and die, leading to a decrease in dopamine levels. PD seems to be hereditary in some patients, and sporadic in most. In 10-20% of cases, people with PD have only one gene alteration [2]. These monogenic genes are named the PARK genes and encode for: alpha-synuclein (α-syn), LRRK2, VPS35, parkin, PINK1, and DJ1.
The most common hallmark of PD is the formation of amyloid aggregates, named Lewy bodies, in the basal ganglia [2]. Lewy bodies were found to mostly contain misfolded α-syn fibril structures [3][4]. The accumulation of these harmful fibril structures, which are resistant to degradation, is thought to lead to impaired nerve cell functioning and cell death. It still remains unclear how these fibril structures form. Finding out how and why α-syn misfolds and aggregates could be the key to understanding PD.
Linking α-syn mutations to aggregation
Recent advances in the cryo-EM workflow, such as better ROI identification in cryo-ET and reduced ice contamination, have made it possible to reconstruct protein structures at near-atomic resolution. This allows researchers to investigate α-syn fibril variants in high resolution (Figure 1a-c), and to find a link between the molecular structure of the α-syn fibrils and their most common mutations [5]. The molecular structure of α-syn proteins consists of three domains, namely an N-terminus, which contains a region where vesicles can bind a central non-amyloid-ß component (NAC) region, which seems to be essential for aggregation to occur; and a C-terminal acidic tail [5][6].
Familial mutations of the SNCA gene are found on the N-terminus, including the most common A53T mutation [5] (Figure 1d). This mutation, which is linked to autosomal dominant, early-onset PD, has been found to promote α-syn fibril formation in vitro [7]. Using cryo-EM, researchers could investigate how and why the A53T mutation promotes fibril formation [8]. They made 3D reconstructions of an Ac-A53T and an Ac-WT α-syn fibril.
By overlaying the near-atomic resolution structures, they found that the A53T mutation changes the paring geometry of the protofilaments, altering the morphology of the fibril and possibly destabilizing it. This might facilitate dissociation and self-propagation of the fibril. Understanding the fibril pathology thus seems to be key to a better understanding of PD. 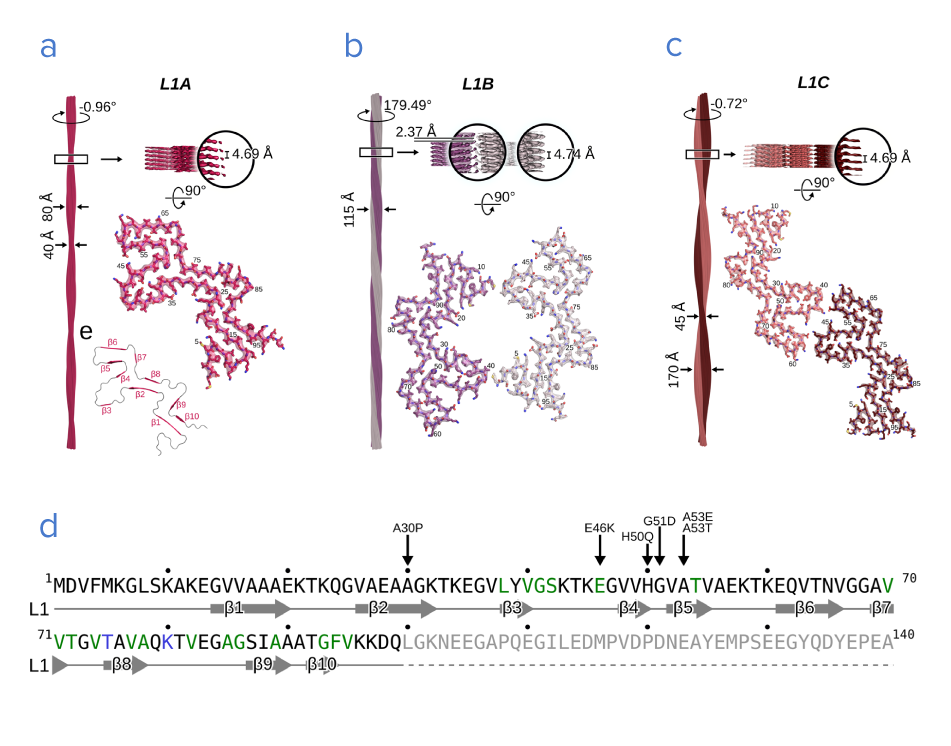 Figure 1: a-c) Three variants of fibril structures (L1A, B, and C) consisting of α-syn proteins. d) Sequence of the α-syn protein. The arrows indicate the most common familial mutations, found in the N terminus. Adapted from [12].
Figure 1: a-c) Three variants of fibril structures (L1A, B, and C) consisting of α-syn proteins. d) Sequence of the α-syn protein. The arrows indicate the most common familial mutations, found in the N terminus. Adapted from [12].
Linking α-syn post-translational modifications to aggregation
Besides genetic mutations, post-translational modifications (PTM) on the α-syn protein have also been associated with Lewy body formation [6]. It was found that phosphorylated Y39 (pY39) in the N-terminus is elevated in sporadic PD patients [9]. The molecular mechanism behind this pY39 mutation was recently investigated using cryo-EM [10].
A reconstruction of the near-atomic structure of the pY39α-syn fibril was made, which revealed that phosphorylation at Tyr39 rearranges the fibril core structure and changes the electrostatic properties of the N-terminus. This research shows that both rearrangements in the N-terminus and PTMs may be important in PD progression.
Linking lipid interactions to aggregation
Apart from direct modifications, lipids interacting with the α-syn protein can also influence its aggregation. Although the biological function of α-syn is not well understood, it has been found to be involved in vesicle trafficking and neurotransmitter release, and it can interact with lipid membranes via its N-terminal. In the presence of lipid vesicles, α-syn displayed enhanced fibrillization [11].
Recently, the molecular interactions between α-syn fibrils and lipids were visualized for the first time [12]. Using cryo-EM, various fibril formations were studied and reconstructions were made of the fibrils surrounding phospholipids (Figure 2).
Surprisingly, the phospholipids were actually seen to rearrange the protofilaments of α-syn and fill the interfibrillar cavity. This suggests that the fibrils induce lipid extraction and possibly disrupt intracellular vesicles. The finding highlights the importance of studying α-syn in its cellular context, and not only as an isolated protein. 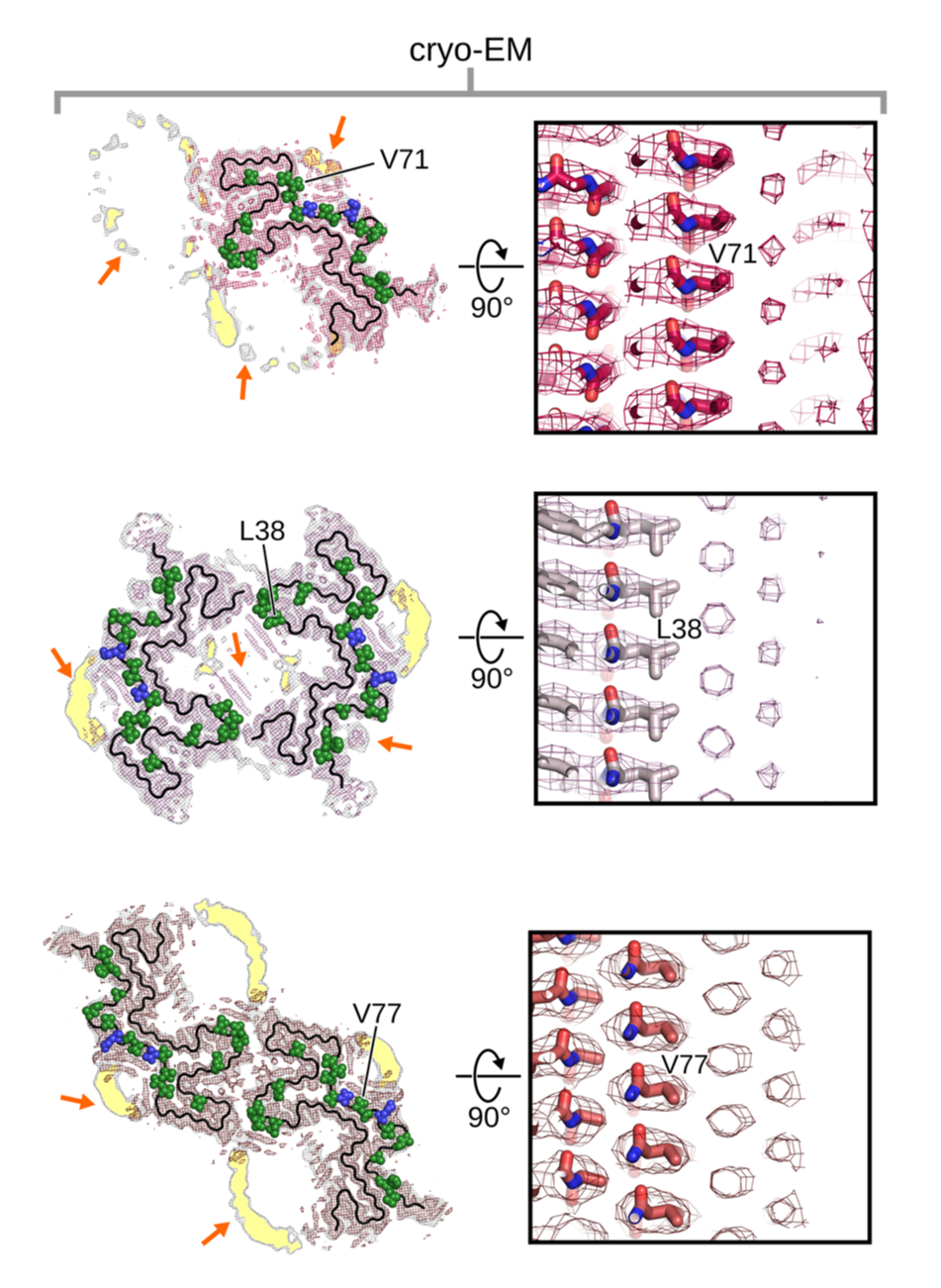
Figure 2: Reconstructions of three fibril variants and surrounding phospholipids using cryo-EM. Adapted from [12].
In conclusion, cryo-EM has changed the way in which we view Parkinson’s disease. It has allowed researchers to zoom in and see the molecular details of α-syn fibrils, investigating its mutations, PTMs, interactions with lipids, and more. This leads to a better understanding of the molecular pathology of PD, and it can provide targets for designing novel PD treatments in the future.
References
- World health organization, official website
- Panicker, N. et al., J Cell Biol 5, 220 (4), (2021)
- Spillantini, M. et al., Nature 388, 839–840, (1997)
- Emamzadeh FN. J Res Med Sci., 21-29, (2016)
- Guerrero-Ferreira, R et al., Current Opinion in Neurobiology 61, 89-95, (2020)
- Creekmore, B.C. et al., Journal of Neuropathology & Experimental Neurology, 80 (6), 514–529 (2021)
- Giasson, B.I. et al., Neuron, 34 (4), 521-533, (2002)
- Sun, Y. et al., Cell Res 30, 360–362 (2020)
- Brahmachari, S. et al., J Clin Invest., 126(8), 2970-2988, (2016)
- Zhao, K. et al., Proceedings of the National Academy of Sciences, 117(33), 20305-20315, (2020)
- Galvagnion, C. et al., Nat Chem Biol 11, 229–234, (2015)
- Frieg, B. et al., Nat Commun 13, 6810, (2022)
.png)