Up until now the structure of leucine-rich repeat kinase 2 or LRRK2 was not yet fully resolved. The paper Watanabe et al. that we discuss in this post explains how using fluorescent light microscopy (FLM), in situ cryo-electron tomography (cryo-ET) and subtomogram averaging analysis reveals the full structure of the LRRK2 complex [1].
We also discussed this paper in our recent journal club. Make sure to watch the recording.
Cellular landscape of LRRK2
In the past decade, the cellular processes and mutation landscapes of LRRK2 have been studied. It was shown that the enzyme plays a role in several pathways such as membrane trafficking , protein synthesis and neurite outgrowth [2]. It also became clear that mutations are mostly located in the catalytic domains and most often lead to hyperactivation of the LRRK2 kinase [3]. Still, how this abnormal activation of LRRK2's kinase is connected to the PD progression remains largely unknown.
Several studies have observed important functions and properties of LRRK2. Wild-type LRRK2 is mostly cytosolic while several of the common PD mutations show filamentous structure formation associated with microtubules in the cell [4,5,6]. Another interesting observation is that the inhibition of the kinase domain of LRRK2 leads to recruitment of LRRK2 on the microtubules in both wild-type and mutated LRRK2 [5]. However, in situ structural information of this important protein is still lacking and therefore also the mechanism of action in the PD landscape.
Cryo-ET imaging to determine the LRRK2 structure
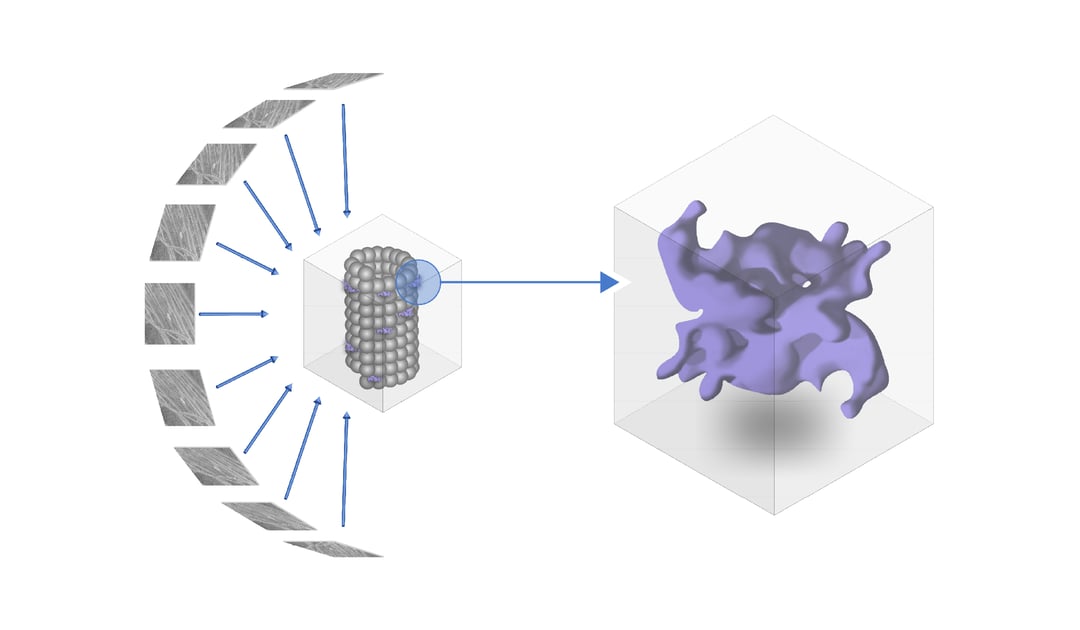
In the article of Watanabe et al., the authors tackle this obstacle by using in situ cryo-ET to determine the structure of human LRRK2 [1]. YFP-tagged LRRK2 that included the I2020T mutation was overexpressed in HEK293T cells. To resolve a high-resolution image of the structure inside the cell, LRRK2 was expressed in HEK293T cells and due to the pathogenic I2020T mutation associates with microtubules.
Cryogenic fluorescence microscopy clearly showed that the microtubules are decorated with LRRK2 proteins. These regions of interest (ROIs) were then thinned down for tomogram acquisition. When analysing the structure of LRRK2 on microtubules, several anomalies are observed when comparing the LRRK2 mutated cell-line with the normal human cell-line. For example, the LRRK2-decorated microtubules grow in a more parallel manner than wild-type microtubules. In addition, significantly more 11 and 12-profilament microtubules were detected next to the 13-profilament microtubule that is normally present in human cells.
To reveal the 3D molecular structure of LRRK2, subtomogram averaging together with integrative modelling was used to gain the in-depth knowledge of the separate domains of LRRK2. The structure was resolved at a resolution of 14 Å. The model was compared to existing models of individual domains of LRRK2 that were previously resolved using X-ray crystallography. One of the most interesting findings was that the I2020T mutation activated the kinase domain of LRRK2 by exposing it to the cytosol. The authors also found that the LRR domain is expressed as hook-like structures stemming from the protomer connecting the two strands of the LRRK2 helices.
Importance of Cryo-ET
Exposing the architecture of LRRK2 in the cellular environment shows that mutations disrupt the normal microtubule-association of the protein. Cryo-electron tomography sheds new light and helps understand the workings of LRRK2, in situ. At Delmic we attempt to contribute to different research fields and develop new imaging solutions to significantly improve and simplify the cryo-ET workflow in the field of life sciences.
Learn more about METEOR, our integrated cryo-FLM solution, and find out how this system can boost your workflow! Or read about our upcoming systems ENZEL and MIMAS!
References
[1] Watanabe, R., Buschauer, R., Böhning, J., Audagnotto, M., Lasker, K., Lu, T.-W., Boassa, D., Taylor, S., & Villa, E. (2020). The In Situ Structure of Parkinson’s Disease-Linked LRRK2. Cell, 182(6), 1508-1518.e16. https://doi.org/10.1016/j.cell.2020.08.004
[2] Kalia, L. V., & Lang, A. E. (2015). Parkinson’s disease. The Lancet, 386(9996), 896–912. https://doi.org/10.1016/s0140-6736(14)61393-3
[3] Steger, M., Tonelli, F., Ito, G., Davies, P., Trost, M., Vetter, M., Wachter, S., Lorentzen, E., Duddy, G., Wilson, S., Baptista, M. A. S., Fiske, B. K., Fell, M. J., Morrow, J. A., Reith, A. D., Alessi, D. R., & Mann, M. (2016). Phosphoproteomics reveals that Parkinson’s disease kinase LRRK2 regulates a subset of Rab GTPases. ELife, 5, https://doi.org/10.7554/elife.12813
[4] Eguchi, T., Kuwahara, T., Sakurai, M., Komori, T., Fujimoto, T., Ito, G., Yoshimura, S.-, Harada, A., Fukuda, M., Koike, M., & Iwatsubo, T. (2018). LRRK2 and its substrate Rab GTPases are sequentially targeted onto stressed lysosomes and maintain their homeostasis. Proceedings of the National Academy of Sciences, 115(39), E9115–E9124. https://doi.org/10.1073/pnas.1812196115
[5] Blanca Ramírez, M., Ordóñez, A. J. L., Fdez, E., Madero-Pérez, J., Gonnelli, A., Drouyer, M., Chartier-Harlin, M.-C., Taymans, J.-M., Bubacco, L., Greggio, E., & Hilfiker, S. (2017). GTP binding regulates cellular localization of Parkinson’s disease-associated LRRK2. Human Molecular Genetics, 26(14), 2747–2767. https://doi.org/10.1093/hmg/ddx161
[6] Kett, L. R., Boassa, D., Ho, C. C.-Y., Rideout, H. J., Hu, J., Terada, M., Ellisman, M., & Dauer, W. T. (2011). LRRK2 Parkinson disease mutations enhance its microtubule association. Human Molecular Genetics, 21(4), 890–899. https://doi.org/10.1093/hmg/ddr526
.png)