Cryo-electron tomography (Cryo-ET) is a powerful imaging technique that enables the visualization of biomolecular structures in their near-native state at subnanometer resolutions. By combining a series of 2D images taken at different tilt angles into a 3D reconstruction, cryo-ET provides unparalleled insights into protein-protein interactions and molecular machinery within cells. This powerful technique has revolutionized structural biology, microbiology, and virology fields by revealing detailed sub-organelle-level information.
Despite the potential of cryo-ET, its adoption in life science research groups is limited due to the challenges associated with sample preparation, particularly the risk of ice contamination affecting sample and data quality.
Challenges in sample preparation
The primary obstacle in cryo-ET sample preparation is the formation of ice contamination, which can significantly decrease the quality of the data. Ice contamination can occur at various workflow stages, from sample preparation to transfer to the TEM. An additional complicating factor is the fact that TEM imaging necessitates sample thicknesses of 150–300 nm, and most samples are too thick. This problem is solved by thinning the cells through cryo-FIB milling. As a consequence, more transfer and handling steps are introduced to the workflow.
Other key pain points in Cryo-ET sample preparation:
- Moisture exposure: During sample preparation and transfer between devices, samples can be exposed to atmospheric moisture, leading to both crystalline and amorphous ice formation.
- Sample targeting: The presence of ice can obscure the region of interest (ROI) and reduce the contrast of TEM images, complicating the identification and targeting of specific molecules.
- Residual moisture in equipment: Even in high vacuum conditions, residual moisture inside equipment like cryo-FIB/SEM chambers can form amorphous ice layers on the sample, affecting data quality and throughput.
Survey results on ice contamination
To better understand the solutions that need to be developed, researchers from Delmic conducted a survey to understand the effects and impact of ice contamination on cryo-ET outcomes.
The survey compiled responses from 84 participants across 16 countries in Europe, North America, and Asia. Here are some of the survey results.
Q: On average, what percent of your cryo samples are ice-contaminated?

Weighted average 36.3%
Q: At what point of the cryo-ET workflow do you realize the samples are ice-contaminated?

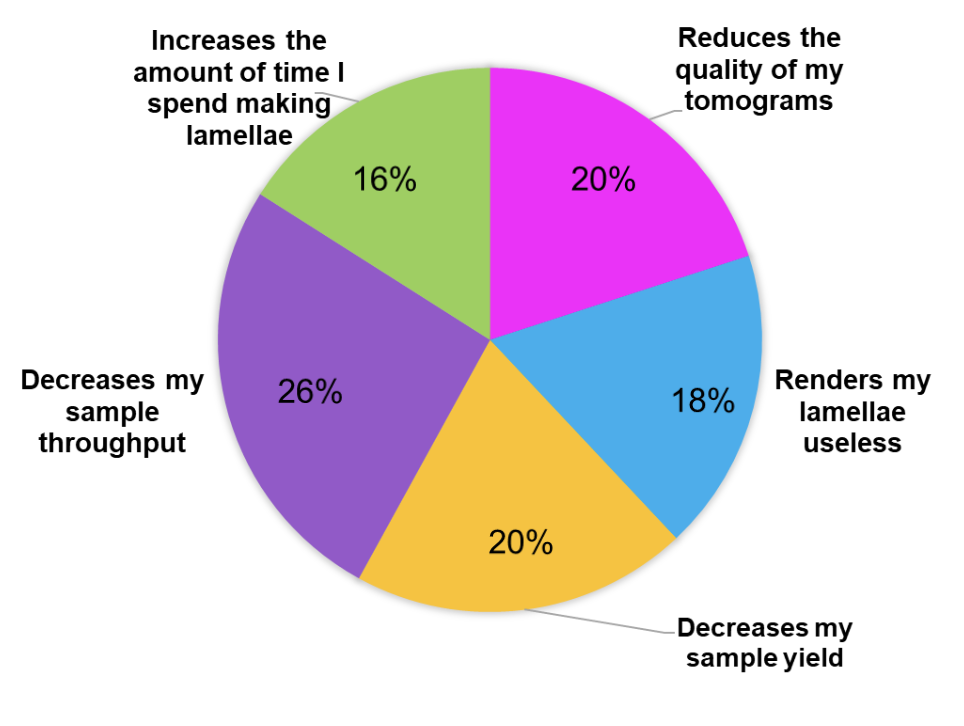
Q: How does ice contamination impact your research?
Q: Which stage of the lamella preparation process is particularly problematic for you?
 Apart from these, the participants encountered various sample problems, including curtaining, leopard ice, non-vitreous ice, ice crystals on milled lamellae, amorphous ice layers, and cracked lamellae.
Apart from these, the participants encountered various sample problems, including curtaining, leopard ice, non-vitreous ice, ice crystals on milled lamellae, amorphous ice layers, and cracked lamellae.
Mitigating ice contamination in Cryo-ET
As you can see from the survey findings, ice contamination remains a significant obstacle in the field of cryo-ET, impacting the quality of tomograms and overall research productivity. Recognizing this challenge, the development team at Delmic collaborated closely with leading academic institutions to develop and commercialize innovative solutions that address various stages of the cryo-ET workflow. This collaborative effort ensures that the research is translated into practical, user-friendly technologies that effectively mitigate ice contamination.
The technologies are as follows:
1. Controlled environment solution
Researchers from the Max Planck Institute for Physiology have identified that ice contamination often originates during sample handling. To tackle this, Tacke et al. [2] developed a specialized controlled environment system that maintains an ultra-low humidity environment by purging it with dry nitrogen gas. This system includes a liquid nitrogen (LN2)-filled preparation table, allowing researchers to handle samples without exposing them to atmospheric moisture, thus preventing crystalline ice formation.

Delmic has commercialized this concept through the CERES Clean Station. It allows for grid handling, clipping, shuttle, and cassette loading within a <1 ppm water enclosure. CERES Clean Station effectively minimizes the risk of crystalline ice contamination on the precious samples and lamellae, thereby providing a reliable and user-friendly solution for researchers.
2. High vacuum transfer system
Ice contamination can also occur during the transfer of cryogenic samples. To mitigate this, a high-vacuum cryo transfer (HVCT) system was developed by Tacke et al [2], which allows for sample transfers under high vacuum conditions. This method minimizes moisture exposure and keeps samples vitrified, thereby reducing crystalline ice formation significantly.

Delmic has integrated this high-vacuum cryo transfer technology into the CERES Vitri-Lock system. By maintaining high vacuum conditions throughout the sample transfer process, Vitri-Lock effectively prevents moisture exposure and subsequent ice contamination. This system is particularly valuable during the transition from and to the cryo-FIB/SEM, as it increases the available transfer time to 30 minutes.
3. In situ cryo-FLM integration
Researchers from Monash University were the first to integrate fluorescence microscopy (FLM) into a cryo-FIB/SEM setup [1], creating an in situ cryo-correlative light and electron microscopy (cryo-CLEM) workflow. This integration reduces ice contamination by eliminating additional transfer steps and avoiding exposure to ambient pressure.

Delmic has commercialized this integrated approach with the METEOR system. METEOR allows for high-vacuum cryo-FLM imaging, minimizing the risk of ice formation during imaging and milling processes. By combining cryo-FLM with cryo-FIB/SEM, METEOR streamlines the workflow and significantly reduces the potential for ice contamination, preserving sample integrity.
4. Cryo-shutter for SEM column protection
To combat amorphous ice growth during SEM imaging, a cryo-shutter cooled by LN2 was developed by Tacke et al [2]. This shutter acts as a protective barrier, shielding the sample from the SEM column and reducing the partial pressure of water vapor, thereby preventing ice formation.

Delmic has translated this protective technology into the CERES Ice Shield. The Ice Shield provides an additional layer of protection during FIB milling by maintaining a low-temperature barrier that prevents amorphous ice growth. This ensures that samples remain uncontaminated during critical imaging steps, enhancing the quality and reliability of cryo-ET data.
Implementing advanced technologies such as Delmic CERES Clean Station, Vitri-Lock, Ice Shield, and Delmic’s integrated FLM METEOR has shown promising results. They aim to mitigate the ice contamination issues in the cryo-ET workflow. Overcoming the major bottlenecks in the cryo-ET workflow is essential to fully unlock the potential of cryo-ET for biomedical research and drug development.
By resolving the inefficiencies caused by ice contamination, the research community can leverage cryo-ET to enhance healthcare outcomes, drive environmental sustainability, and contribute to societal well-being. Therefore, there is a pressing need for a significant reduction in ice contamination throughout the cryo-ET workflow to realize the full potential of this technique for biomedical research and drug development.
Based on the study here - The Undesirable Effects and Impacts of Ice Contamination Experienced in the Cryo-Electron Tomography Workflow and Available Solutions
References:
- Gorelick, S et al. , eLife 8 (2019)
https://doi.org/10.7554/eLife.45919 - Tacke, S et al. , J Struct Biol 213 (2021) https://doi.org/10.1016/j.jsb.2021.107743
.png)







