Cathodoluminescence can be imaged separately from other, often complementary, types of emissions, and is used for advanced investigation of material properties. One such complementary emission form is photoluminescence. But what is the difference between these two types of emissions?
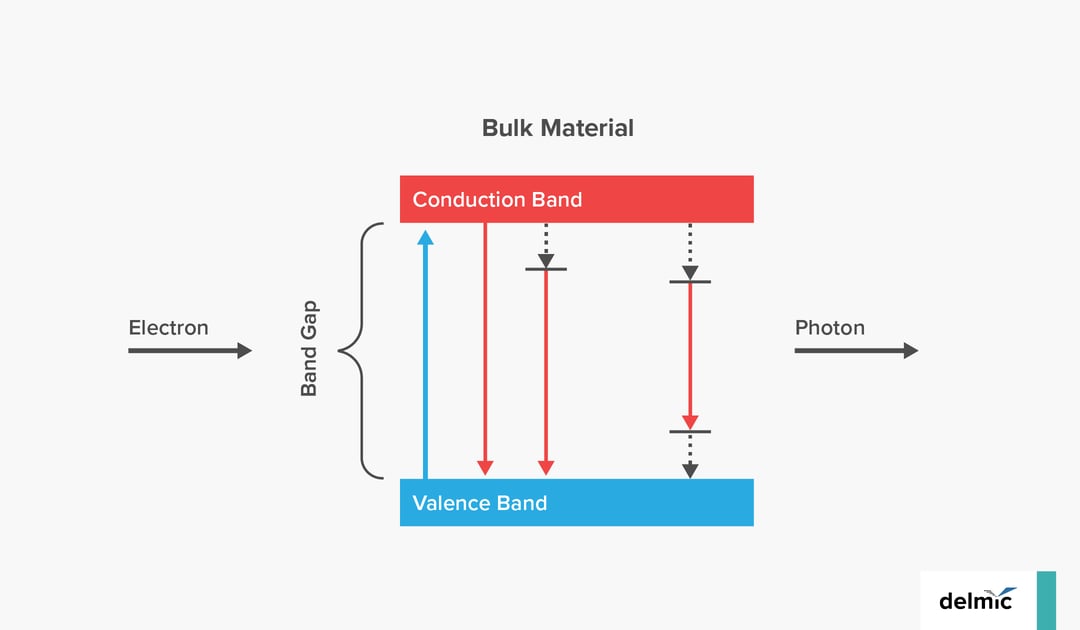
Cathodoluminescence (CL) is, in broad terms, the process of light emission from a material as a result of excitation by electrons. In particular, incoherent CL emission takes place when the electron beam excites quantum mechanical transitions in the material, promoting electrons from the valence band to the conduction band. From there, the electron can decay radiatively, with the emission of a photon. In bulk materials, the excited electrons decay directly to the valence band, emitting a photon. In quantum-confined structures, the bands themselves can become quantized, and this discretization is visible in the CL emission. So CL provides a signature of these processes. There is no fixed phase relation between the incoming electron and the outgoing photon, and the emission is typically unpolarised and isotropic. Although this sounds a lot like photoluminescence (PL) or fluorescence, the two processes are significantly different as well. Materials can also show both CL and PL.
PL is the process of light emission as a result of excitation by photons. Since electrons are massive and carry more momentum, the selection rules for CL emission are different from those for PL. The light sources, often lasers, used for PL excitation have a narrow energy range and therefore probe specific transitions. Excitation by electrons, on the other hand, is broadband, allowing inspection from the deep UV to the IR. Furthermore, due to the high energy of the electrons, multiple excitation paths are available, resulting in different relaxation pathways with corresponding CL signatures. The CL excitation process can also be more efficient than that of PL as a result of the high electron energy, allowing coupling to different intermediate states in the material, such as defect states.
Both of these techniques can be beneficial for uncovering valuable information about material properties.
.png)