This is due to the fact that samples thicker than 10 μm cannot be fully frozen by plunge freezing. This problem can be solved by employing high pressure freezing (HPF) technique for thicker samples. Unfortunately, HPF samples thicker than ~50 μm can still not be thinned by standard FIB milling since the ion milling speed and focus depth are limited.
There are several methods available to overcome this problem and extend cryo-FIB milling to thicker samples. One of the suggested methods is ‘on the grid lamella milling’ which was tested by Harapin et al. on HPF C. elegans worms[1]. The milling of the worms took approximately 30 hours and they had to use organic solvents to be able to freeze the worms without embedding them in a bulk sample. Another approach is to first trim the HPF cells using cryo-ultramicrotomy before FIB milling. This method was successfully applied by Hsieh et al. on muscle tissue from toadfish. It was however difficult to also employ cryo-FLM to target specific structures within the tissue[2].
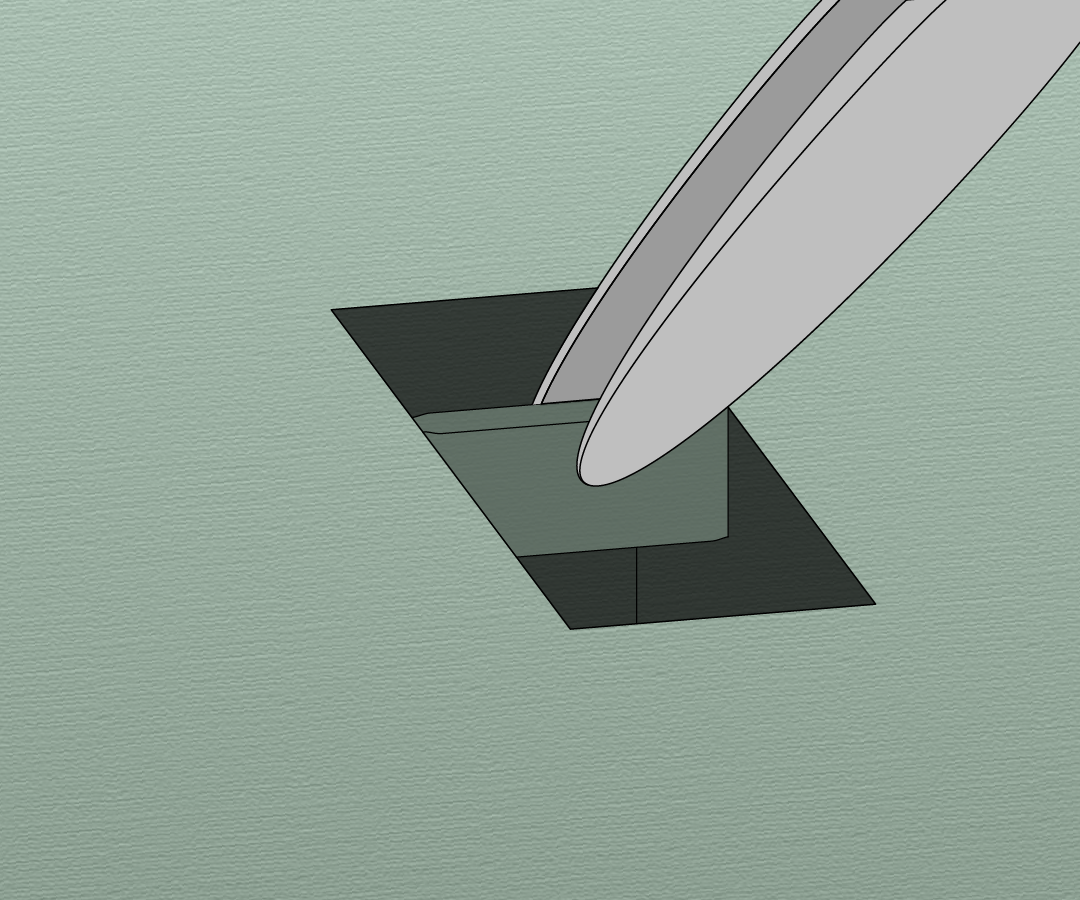
Another approach is in situ lift-out, in this method a small section is extracted from the bulk material and transferred to a TEM grid for final FIB milling. This method is well established at room temperatures but requires some adjustments at cryo-temperatures. The feasibility of this approach under cryo conditions was established by Mahamid et al.[3]. They first used cryo-ultramicrotomy on HPF C. elegans worms to expose the surface of the sample and then cryo-FLM to find the region of interest (ROI). In the FIB/SEM the ROI was found back, a ~5 μm thick section was cut free by the FIB, lifted out by a micromanipulator and transferred to a TEM grid where the final thinning took place.
Recently Schaffer et al. improved this method by using a cryo-gripper tool to lift-out sections from HPF C. elegans worms[4]. They were able to resolve the structure of cytosolic 80S ribosomes within C. elegans with resolutions up to 11.8 Å. There is however still a lot of room for improvement since the production of a single cryo lift-out lamella took up to 10 hours and the success rate was smaller than 20%.
At Delmic we are currently developing an integrated FLM solution called METEOR that could simplify this technique. The integration will reduce the number of transfer steps and reduce surface contamination that can be introduced during transfers. Moreover, having an FLM inside the FIB/SEM chamber allows FLM imaging of the extracted volume to guide the final FIB milling step.
Cryo-FIB lift-out is a very promising technique that allows the study of in situ structures in multi cellular organisms or tissues. There are however several technological advances necessary to simplify the technique and make it widely adopted. Delmic is proud to be contributing towards the technological advances in this field.
References
[1] Harapin, J. et al. Structural analysis of multicellular organisms with cryo-electron tomography. Nature Methods 12, 634–636 (2015).
[2] Hsieh, C., Schmelzer, T., Kishchenko, G., Wagenknecht, T. & Marko, M. Practical workflow for cryo focused-ion-beam milling of tissues and cells for cryo-TEM tomography. Journal of Structural Biology 185, 32–41 (2014).
[3] Mahamid, J. et al. A focused ion beam milling and lift-out approach for site-specific preparation of frozen-hydrated lamellas from multicellular organisms. Journal of Structural Biology 192, 262–269 (2015).
[4] Schaffer, M. et al. A cryo-FIB lift-out technique enables molecular-resolution cryo-ET within native Caenorhabditis elegans tissue. Nature Methods 16, 757–762 (2019).
This work is supported by the European SME2 grant № 879673 - Cryo-SECOM Workflow
.png)