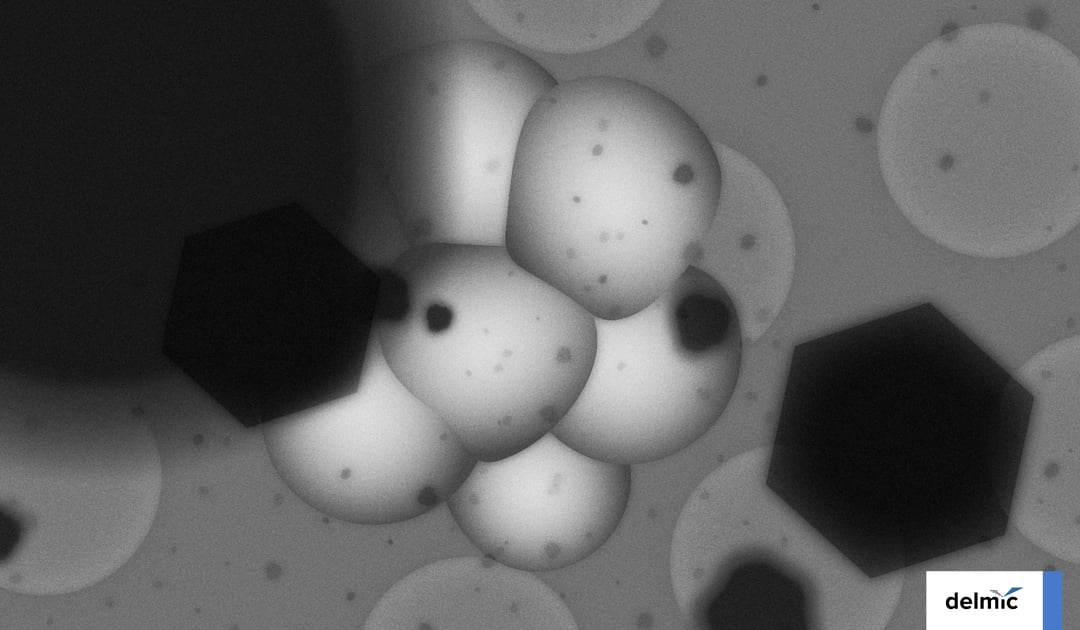
Single particle cryo-electron microscopy (cryo-EM) is a powerful method to determine near-atomic structures of macromolecular complexes. In recent years this technique resolved many structures with a sub 4-Å resolution such as the Zika virus [1], hemoglobin [2], and ribosomes in solution [3].
To prepare a sample for cryo-EM, a purified protein solution is first applied on a TEM grid and plunge frozen. Next, the grid is clipped in an AutoGrid and loaded in the cassette. The cassette is then transferred to the NanoCab and loaded in the cryo-TEM for data collection. To keep the sample vitrified it is submerged in liquid nitrogen during the entire sample preparation process. During all the preparation steps there is a chance of ice contamination of the samples reducing the quality of the TEM data or even rendering the sample useless [4]. Amorphous ice can form on the sample due to the moisture in the atmosphere. To reduce this, the steps described above should ideally be carried out in a dehumidified room. It is also recommended to always use clean, freshly decanted liquid nitrogen since ice crystals can form in liquid nitrogen that can land on top of the sample. Moisture present on the tools used for clipping and sample handling can also lead to ice crystals forming on the sample, they should therefore always be heated and dried before use. It is also recommended to wear a face mask to avoid warm moist breath on the samples that can lead to devitrification or ice contamination.
However, when taking all these measures into account, ice contamination still cannot be completely prevented. According to our survey, on average 35% of the cryo samples are ice contaminated. Ice contamination can prevent good data from being acquired from the precious cryo-samples. Since cryo-TEM instrument time is costly and the sample preparation procedure is also very time-consuming, ice contamination is a very costly problem.
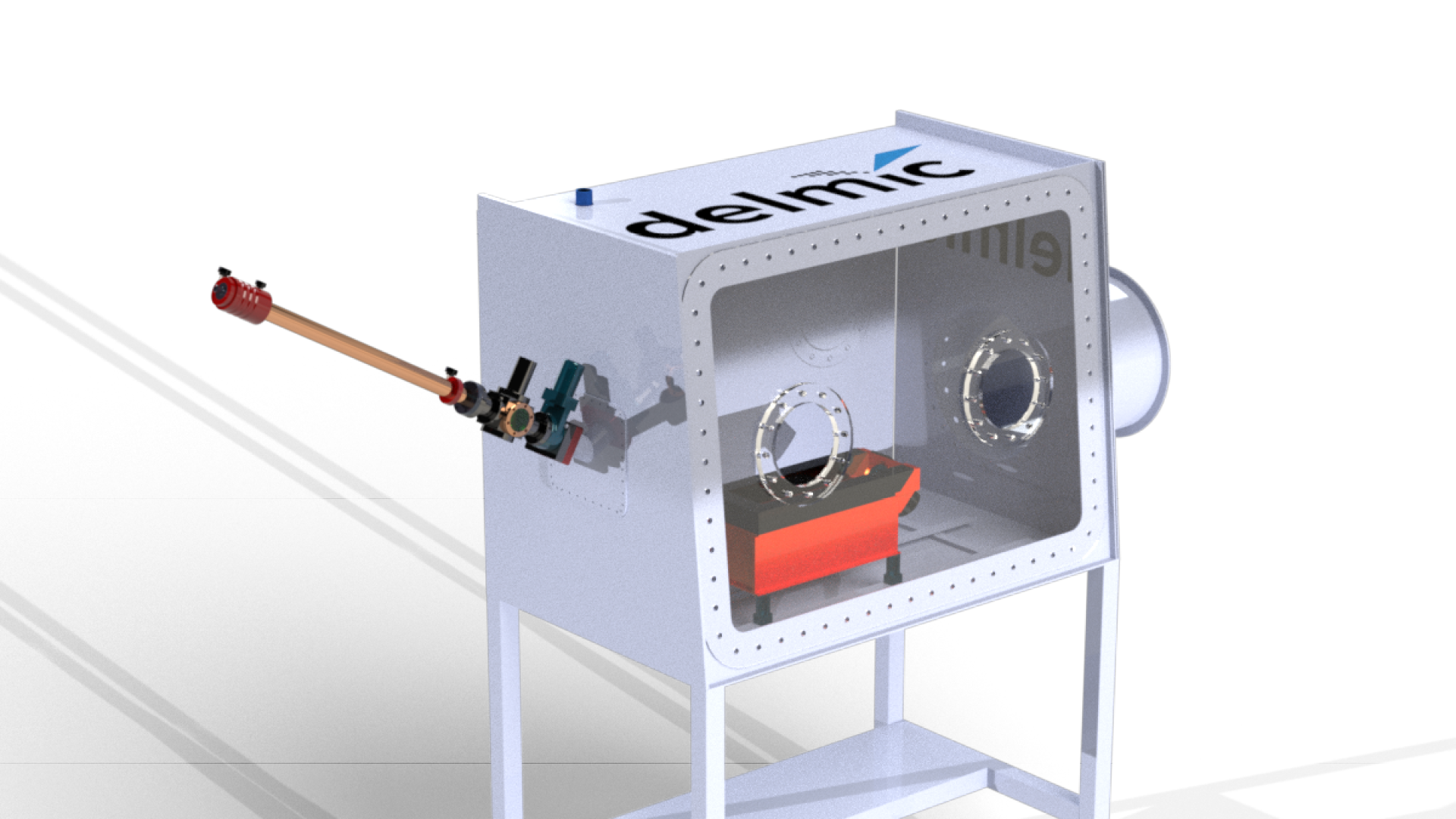
To solve this problem we developed the CERES Clean Station in collaboration with the Max Planck Institute of Molecular Physiology [5]. The CERES Clean Station constitutes a dry nitrogen gas-purged glove box and load-lock to provide a <1% humidity environment. It is therefore a more effective and environmentally friendly solution than a low-humidity room.
Inside the CERES Clean Station is a liquid nitrogen-cooled preparation table providing a safe and user-friendly sample preparation platform. The preparation table consists of two modules specifically designed for C-clipping and cassette loading. The loaded cassette can be transferred to the NanoCab through an airlock to minimize the introduction of moisture in the clean station.
After use, the tools and preparation station can be easily heated to remove any moisture to prepare for the next user. The CERES Clean Station thus provides a user-friendly and ice-contamination-free environment to prepare samples for high-resolution cryo-EM data acquisition.
To learn more about our CERES Ice Defence system, please visit the product page.
References
[1] Sirohi, D. et al. The 3.8 Å resolution cryo-EM structure of Zika virus. Science 352, 467–470 (2016).
[2] Khoshouei, M., Radjainia, M., Baumeister, W. & Danev, R. Cryo-EM structure of haemoglobin at 3.2 Å determined with the Volta phase plate. Nature Communications 8, (2017).
[3] Bai, X. C., Fernandez, I. S., McMullan, G. & Scheres, S. H. W. Ribosome structures to near-atomic resolution from thirty thousand cryo-EM particles. eLife 2013, (2013).
[4] Thompson, R. F., Iadanza, M. G., Hesketh, E. L., Rawson, S. & Ranson, N. A. Collection, pre-processing and on-the-fly analysis of data for high-resolution, single-particle cryo-electron microscopy. Nature Protocols 14, 100–118 (2019).
[5] Tacke, S. et al. A streamlined workflow for automated cryo focused ion beam milling. bioRxiv 2020.02.24.963033 (2020) doi:10.1101/2020.02.24.963033.
This work is supported by the European SME2 grant № 879673 - Cryo-SECOM Workflow.
.png)