Bacterial microcompartments are widely known for their essential role in catabolic processes. Among them, carboxysomes (CBs) are responsible for CO2 fixation. In this process, carbonic anhydrase and Rubisco (the primary enzymes for CO2 fixation) are encapsulated in the proteinaceous shell of the carboxysome (Figure 1). This compartmentalization increases the local concentration of CO2 inside the microcompartment and thereby improves the turn-over of the Rubisco enzyme and on-target catalysis [1].
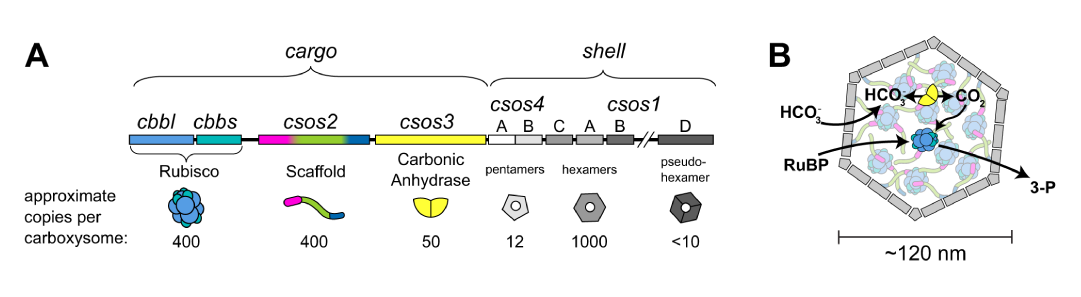
Figure 1: Schematics of the interior structure of the carboxysome. Taken from [1].
Using cryo-ET to resolve the structure of Rubisco
While carbonic anhydrase is mainly responsible for concentrating CO2, Rubisco is the primary enzyme for carbon fixation. Therefore, Metskas and coworkers [2] opted to understand how Rubisco’s assembly relates to its activity within the microcompartment. To this end, they purified α-carboxysomes containing different concentrations of Rubisco from the Halothiobacillus neapolitanus species. Subsequently, they used cryo electron tomography (cryo-ET) and subtomogram averaging to resolve the structure of Rubisco and investigate its 3D organization and packing within the carboxysome.
Cryo-ET is known for its unmatched power in revealing rare cellular events. With cryo-ET, scientists are able to investigate the ultrastructure of cellular components with sub-nanometer resolution and make accurate hypotheses on the intricate interplay between them in their near-native state.
Concentration-dependent organization of Rubisco
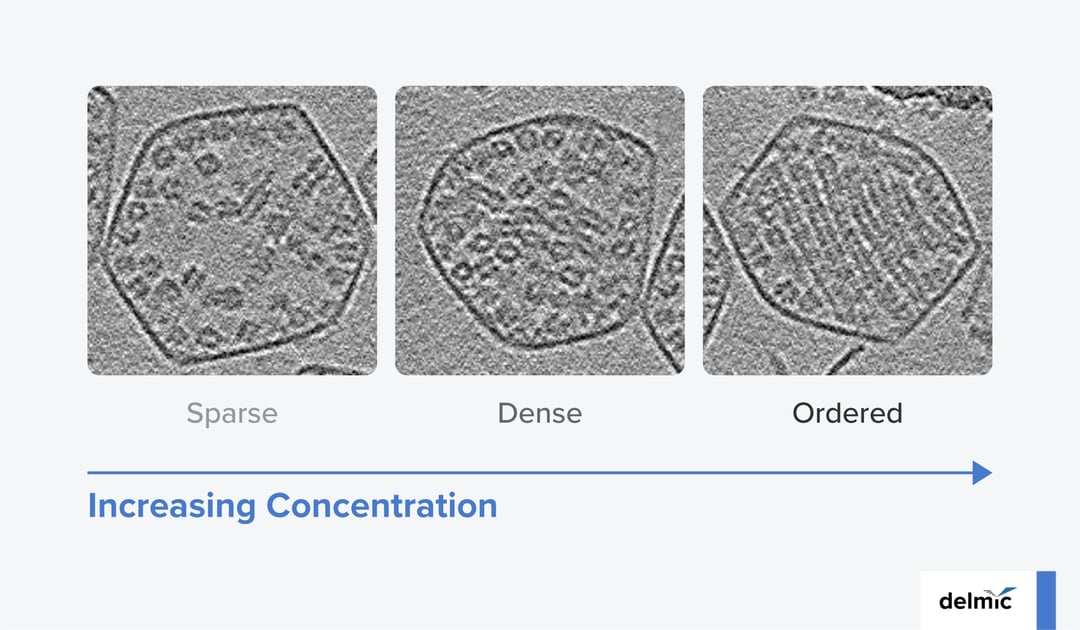
Resolving the ultrastructure of Rubisco revealed a symmetric structure for this protein, hinting towards a natural tendency for fibril formation. However, further high-resolution investigations showed that the organization of Rubisco inside the microcompartments is in fact concentration dependent (Figure 2). For low concentrations, a sparse packing configuration for Rubisco is observed which helps keeping the active sites of the enzyme open. However, for high enough concentrations, Rubisco polymerizes into fibrils and exhibits a dense packing configuration. Finally, in the majority of the carboxysomes, these fibrils further pack into an intertwined lattice and form a tight, ordered packing configuration.
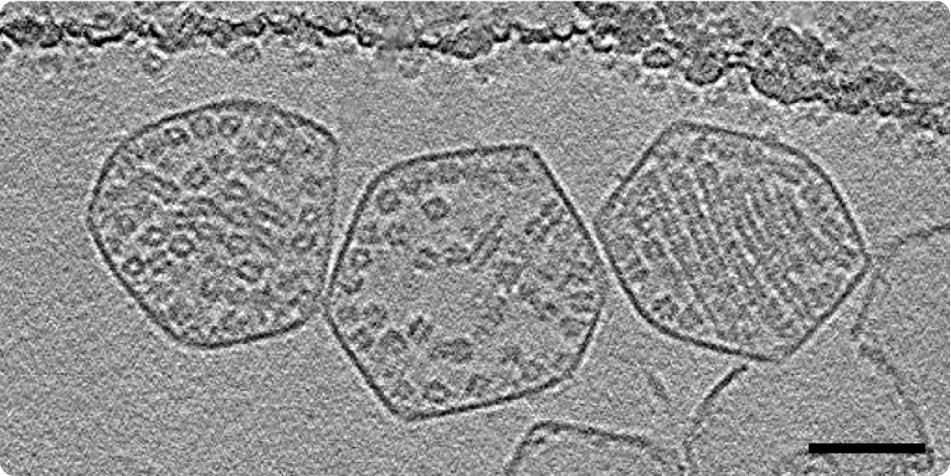 Figure 2: Three carboxysomes with three different packing behaviors: (left to right) Dense, sparse, ordered. Scale bar: 50 nm. Taken from [1].
Figure 2: Three carboxysomes with three different packing behaviors: (left to right) Dense, sparse, ordered. Scale bar: 50 nm. Taken from [1].
Both polymerization and twisted lattice configuration are effective methods to prevent the crystallization of the enzyme at higher concentrations and counteract the steric limitations it poses on enzyme functionality. The resolved ultrastructure of Rubisco subunits in a fibril, further highlighted the weak interaction between Rubisco-Rubisco, explaining the wobbly nature of the fibril and indicating the possibility for reorganization of the interior of the CB. This was also verified in parallel by Ni et al. [3] where they treated the purified CBs with super-physiological calcium concentrations and observed that the fibrils formed lattices with a different symmetry.
To take into account the possible constraints that purification imposes on heterogeneous structures, Metskas and coworkers additionally studied the structure of CBs within intact cells. The in-situ investigations also revealed the dense and ordered packing configurations of Rubisco inside the native CBs, with the addition of elongated layered configurations that were lost during purification.
Exploring large areas of heterogeneous samples is essential
The heterogenous structure and composition of microcompartments such as carboxysomes emphasizes the need to be able to screen large areas within one sample as well as the need to investigate multiple samples with different origins. While carboxysomes from one bacterial species encapsulate Rubisco in concentric circles [3], CBs from other species [2] encapsulate Rubisco in intertwined spirals. As a result, capturing all the different organizations and assembly patterns is not possible with exploring small areas of samples with single origins.
As we can see, the applications of cryo-ET are stretching beyond structural investigations and towards revealing the assembly principles and mechanisms behind cellular components. These assembly mechanisms could be invaluable in the fields of synthetic biology and metabolic engineering where scientists are nowadays trying to investigate the use of engineered organisms for sustainable chemical productions and other biological applications [4]. Here at Delmic, we strive to contribute to the rapid expansion of these fields by developing solutions to optimize cryo-ET workflows. Our products aim to make microscopy easy and accessible to everyone and thereby shift the efforts from laborious workflows to immense scientific discoveries.
References
- Liu, L. N., Trends Microbiol. 30, 567–580, (2022).
- Metskas, L. A. et al., Nat. Commun. 13, 4863, (2022).
- Ni, T. et al., Nat. Commun. 13, 4299, (2022).
- Treece, T. R. et al., Front Bioeng Biotechnol 10, (2022).
.png)