Correlative light and electron microscopy (CLEM) is a set of techniques that integrate information from images acquired through both light and electron microscopy. In order to understand the significance of cryo-CLEM, we will first delve into the application of cryo-electron microscopy (EM) in life sciences.
Major advances in cryo-electron microscopy (cryo-EM) have given rise to the development of diverse cryo-EM techniques designed for imaging biological samples. Cryo-EM provides high-resolution imaging for all objects within a sample and has led to numerous breakthroughs in life sciences. Nevertheless, it also poses certain challenges, some of which can be solved through the complementary use of light microscopy (LM) [1].
Combining cryo-ET and cryo-FM
An upcoming technique in cryo-electron microscopy is cryo-electron tomography (cryo-ET). Cryo-ET allows for the imaging of macromolecular complexes and proteins within their native cellular environment. The process involves vitrification of the sample, thinning it at the region of interest (ROI), and acquiring a series of image projections using a transmission electron microscope (TEM) at a range of angles from -60° to +60°.
The resulting tilt series can be used to reconstruct the 3D structures of, for example, intracellular organelles and protein complexes with subnanometer resolution through a process called subtomogram averaging. Cryo-ET has provided novel insights into the ultrastructures of cells and protein-protein interactions in both healthy and diseased tissues [2].
Despite the valuable insights offered by cryo-ET, the technique has yet to be widely adopted. One contributing factor is its lengthy and laborious workflow. Prior to conducting TEM imaging, the sample has to be thinned sufficiently to let electrons pass through.
The thinning process typically involves using a focused ion beam (FIB) inside the chamber of a scanning electron microscope (SEM), which mills the ROI to produce a thin lamella suitable for TEM imaging. Accurate localization of the ROI in the ice, as well as monitoring of the ROI during milling, is crucial for obtaining useful lamellae [1][3]. This is where light microscopy comes into play.
Light microscopy offers the advantages of rapid screening and fast detection of objects within a sample through labeling strategies. The discovery of fluorescent proteins, in particular, has significantly simplified the labeling and imaging of molecules of interest using fluorescence microscopy (FM).
Recognizing the potential, researchers in cryo-ET realized that by combining cryo-ET and FM, they would be able to detect the molecules of interest as well as capture high-resolution images of them.
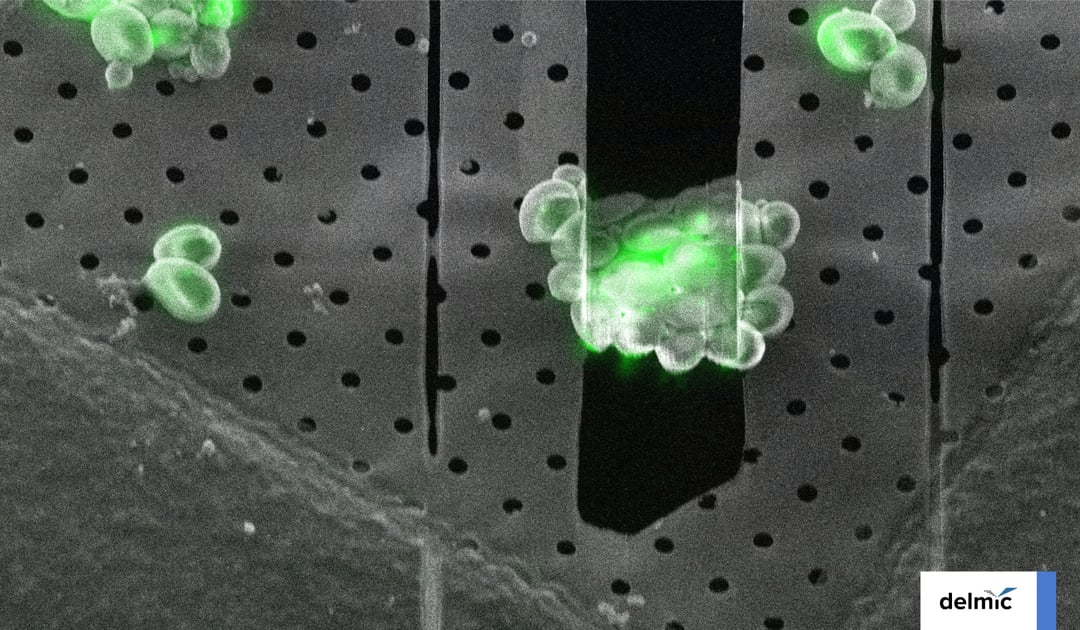
In response to this demand, researchers engineered GFP so that it can retain its fluorescence signal even after the sample is frozen [4]. This innovation made it possible to locate the region of interest in the sample using cryo-FM, and mill at that specific location in the FIB/SEM (Figure 1).
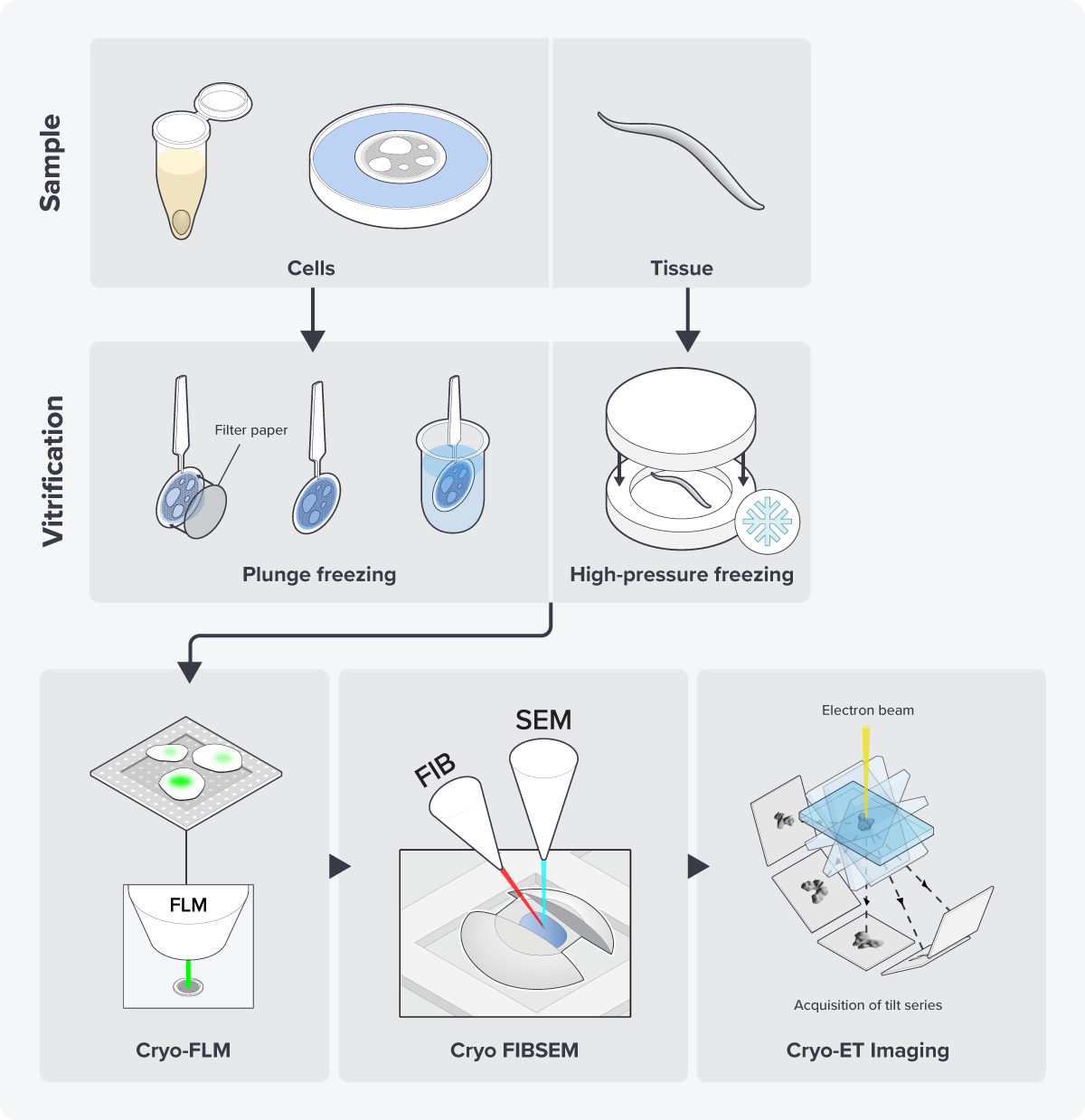
Figure 1: The cryo-ET workflow including cryo-FLM imaging to locate the region of interest before cryo-FIB milling.
So, by combining these techniques, researchers use the advantages of each, addressing challenges in one technique with the strengths of the other. This way, you could say they have the ‘best of both worlds’, encompassing functional information from FM and the structural information from EM. This specific CLEM workflow is now regularly used for cryo-ET research [3][5] (Figure 2).
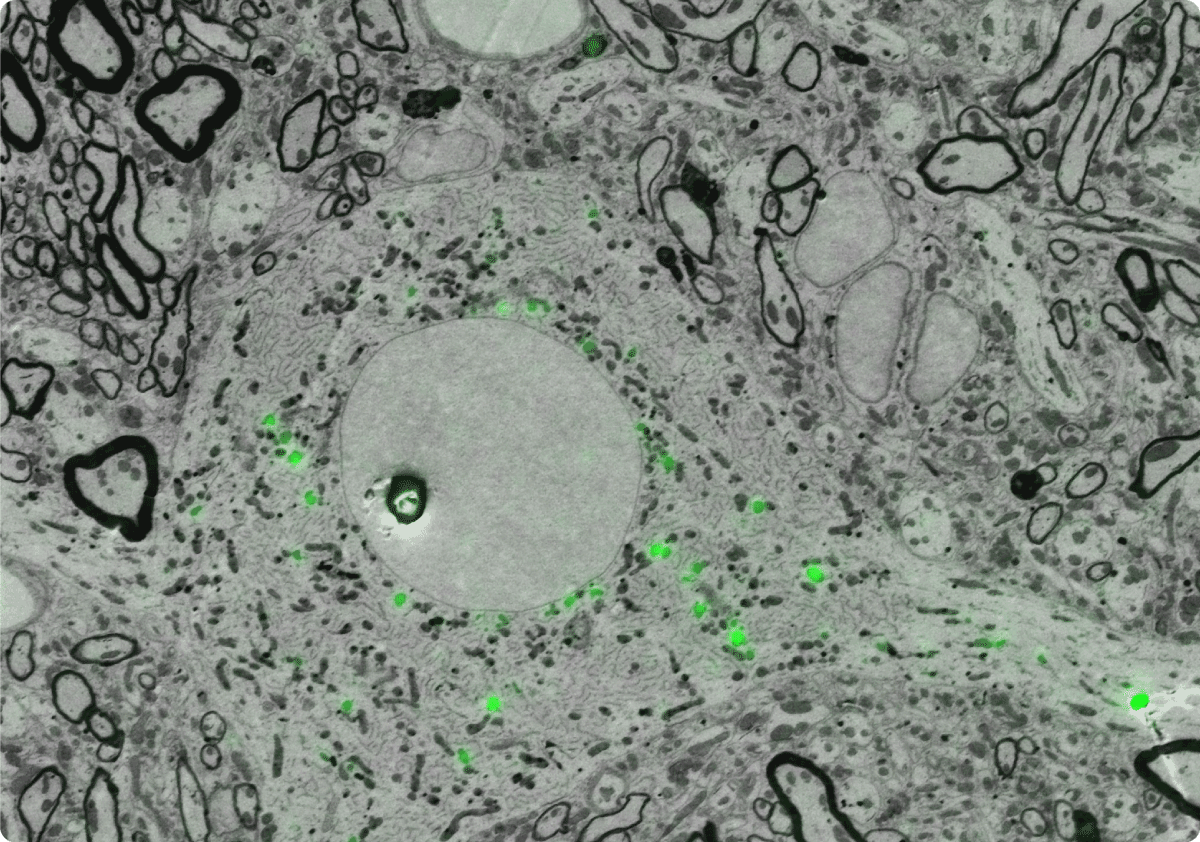
Figure 2: Example of an EM image correlated with the FLM image, showing the ultrastructural details of the cell, as well as the location of the fluorescent proteins.
Future outlook
The combination of cryo-ET and FM has resulted in groundbreaking insights across various research fields, including neurology, cell biology, and virology [2][5][6]. In recent years, solutions have also been developed to make the cryo-CLEM workflow easier and more efficient [7].
The correlation of FM and EM images poses challenges due to the resolution difference between EM and FM techniques, and the extensive sample preparation adds to the complexity. To tackle the resolution gap, researchers are implementing super-resolution FM techniques [5][7].
The lengthy workflow is mainly attributed to the transfer steps between the FIB-SEM and FM, which can lead to ice contamination and devitrification. To minimize this and simplify image correlation, Delmic has developed METEOR, a fluorescent light microscope designed for integration into a FIB-SEM [3].
These innovative techniques and advancements in both EM and FM contribute to the easier implementation of the CLEM workflow in EM research, making cryo-ET a more attractive technique. Are you curious how the cryo-CLEM workflow can be applied to imaging eGFP-tagged proteins in yeast cells? Read it in our application note below!
References
[1] Guaita, M. et al., Current Opinion in Structural Biology 77, 102484 (2022)
[2] Turk, M. et al., FEBS Lett 594, 3243-3261 (2020)
[3] Smeets, M. et al., Microscopy Today, 29(6), 20-25 (2021)
[4] Van den Dries, K. et al., FEBS Lett 596, 2486-2496 (2022)
[5] Jun, S. et al., Protein J. 38, 609–615 (2019)
[6] Wang, S. et al., Biophys Rep 3, 8–16 (2017)
[7] Cortese, K. et al., The journal of histochemistry and cytochemistry 57, 1103-12 (2009)
.png)