Cryo-electron tomography (Cryo-ET) has transformed our understanding of the structural organization and functioning of proteins and macromolecules in their near-native state. In cryo-ET, a series of two-dimensional images are captured from various angles of the sample using a transmission electron microscope (TEM). These images are then used to generate three-dimensional tomograms, which provide valuable insights into the architecture of proteins and macromolecular complexes in situ.
In TEM imaging, the ideal sample thickness is below 300 nm [1]. As a result, most samples need to be thinned, which is typically achieved using cryo-focused ion beam (FIB) and scanning electron microscopy (SEM) instruments. By precisely milling the sample with an ion beam, a thin and electron-transparent lamella is generated, which can be imaged with the TEM. This method is also referred to as on-the-grid cryo-lamellae preparation.
Cryo-ET for multicellular organisms and tissues
While this sample preparation method works well for samples containing single cells, it is not suitable for thicker samples such as small model organisms or tissue. However, these thicker samples are of great importance for studying rare protein complexes and organelles in situ, particularly in the fields of cell and developmental biology [1]. Moreover, these thicker specimens have greater physiological relevance compared to single cells.
Sample preparation for these thicker samples is challenging because cryo-ET samples must be rapidly frozen to prevent the formation of ice crystals, which becomes difficult for samples thicker than 10 µm [1]. Therefore, high-pressure freezing (HPF) is used to freeze thick samples quickly, embedding the sample in a thick slab of ice [2]. There are currently two methods available to extract thin lamellae from this slab of ice: the Waffle method, and cryo-FIB lift-out.
In this blog post, our focus will be on the lift-out method. In lift-out, bulk sample areas are cut free with the ion beam and then transferred to a separate grid for milling. This method is commonly used in material science [3]. The advantages of this approach are:
- Free of artifacts
- Doesn't require long and impractical milling times.
Although first deemed impossible, recent advancements in lift-out techniques have made it possible to apply this method to cryogenic samples as well. Below, we explore the latest research on the cryo-FIB lift-out technique and its promising applications.
Modified nanomanipulators and tweezers
One of the first cryo-FIB lift-out methods was reported by Rubino et al. in 2012 [4]. They made modifications to a nanomanipulator with a tungsten needle by incorporating an insulation component and connecting it to a cooled anti-contaminator using copper braids. Trenches were first milled on either side of the area of interest to cut out the thick lamella. They deposited a layer of Pt precursor to weld together the nanomanipulator with the lamella (Figure 1).
Subsequently, the thick lamella or ‘chunk’ was moved to a TEM grid for milling. To leave the lamellae attached to the grid, they milled away the tip of the nanomanipulator. They then used a custom-built transfer station to transfer the thinned lamella from the FIB-SEM support to a cryo-TEM holder.
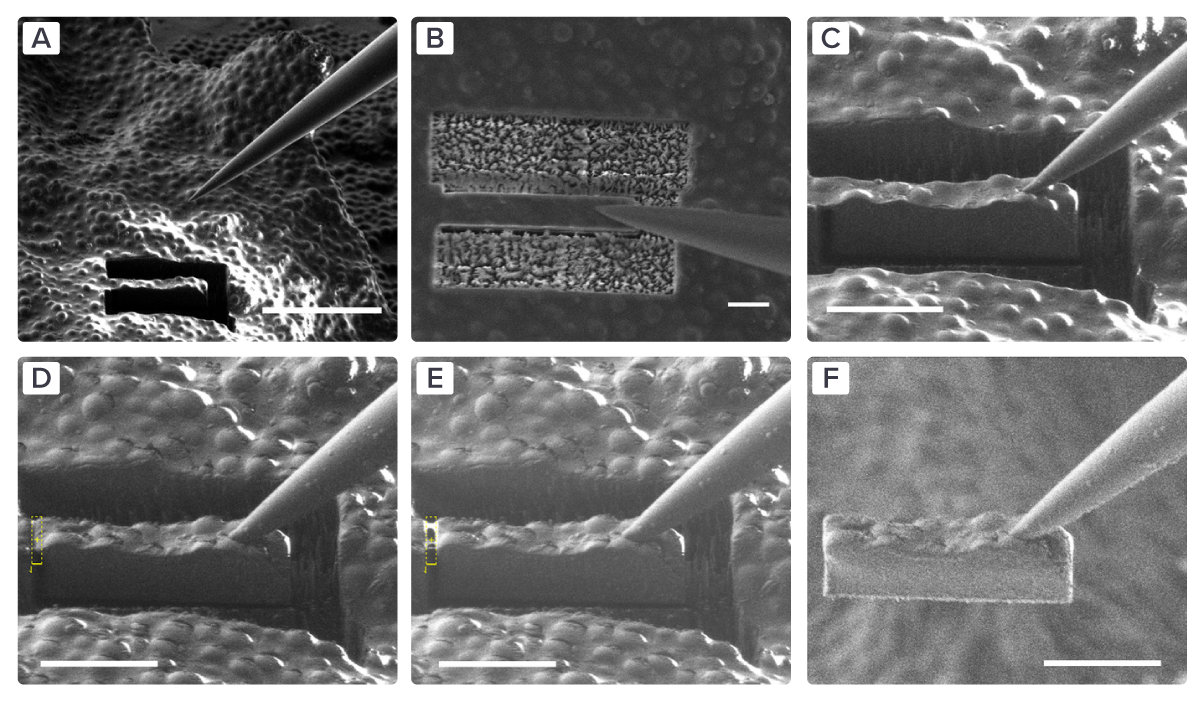 Figure 1: EM images of the complete lift-out procedure in six steps. A-B) The micromanipulator approaches the sample containing milled trenches and positions between the trenches. C-D) The needle makes contact with the lamella, and they are attached to each other. E) The connection between the lamella and the bulk sample is milled away. F) The lamella is lifted out of the bulk sample. Taken from [3].
Figure 1: EM images of the complete lift-out procedure in six steps. A-B) The micromanipulator approaches the sample containing milled trenches and positions between the trenches. C-D) The needle makes contact with the lamella, and they are attached to each other. E) The connection between the lamella and the bulk sample is milled away. F) The lamella is lifted out of the bulk sample. Taken from [3].
Building upon this method, researchers started to use cryo-fluorescence light microscopy (cryo-FLM) to target their area of interest within the bulk sample [1][5]. With the development of the integrated fluorescence light microscope, it is now possible to identify and correlate the target sites in the FIB/SEM itself without risky transfer steps [6].
To lift out the thick lamellae, researchers now either use a modified micro- or nanomanipulator, similar to the nanomanipulator described in the previous section, or they use a cooled tweezer-like gripper with two tungsten probe tips [1][5][7]. The advantages of the nano- and micromanipulator are the ease of interchanging tungsten needles, which is relatively less costly than the gripper system, and the fact that it requires less trench milling due to its slimmer design [7]. Both needles and grippers are commercially available [8][9].
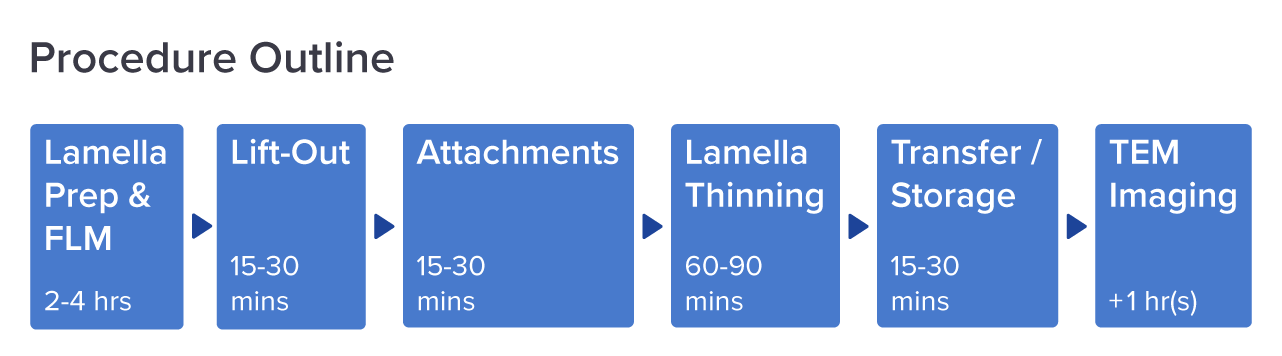 Figure 2: Workflow diagram for cryo-EM imaging using cryo-FIB lift out. The time spent is estimated per step. Adjusted from [3].
Figure 2: Workflow diagram for cryo-EM imaging using cryo-FIB lift out. The time spent is estimated per step. Adjusted from [3].
Pt-free lift-out techniques
The aforementioned methods described the deposition of a layer of Pt precursor to attach the sample to the lift-out device. However, in cryogenic conditions, Pt undergoes desublimation, resulting in the addition of a layer to the sample's surface during each lift-out round. Consequently, the lamella becomes too thick after a couple of transfers [9]. Recently, researchers have developed an alternative approach that eliminates the need for Pt in every lift-out round [10][11].
In this new method, they used an ion beam to sputter material at the interface between the lamella and the grid or micromanipulator. As a result, atoms in the material are sputtered from the surface, and some of these atoms subsequently reattach, a phenomenon known as redeposition.
This redeposition process effectively attaches the lamella to the micromanipulator needle or the grid. After completing all transfer steps, a Pt layer is deposited on the grid to protect lamella preparation. By using this redeposition principle, it is possible to achieve Pt-free lift-out using either the micromanipulator or the tweezer-like gripper.
Multiple lamellae from one lift-out volume
One drawback of using lift-out in cryo-ET, in comparison to various volume electron microscopy techniques, is the loss of a large portion of the volume (± 99%). However, cryo-ET has the advantage of achieving the highest resolution at a similar sample preservation state.
In a recent preprint, researchers introduced a technique called ‘serial lift-out’, which enables the extraction of a series of lamellae from a single lift-out volume [6]. This technique provides additional contextual information about the sample and increases the overall throughput.
The researchers tested the effectiveness of serial lift-out on an HPF sample containing multiple C. elegans larvae. They used cryo-FLM to precisely locate and cut out samples containing an entire larva. A cut-out sample was then attached to a lift-out needle through a copper block. Subsequently, it was transferred to a grid with rectangular meshes, where the bottom part was attached to one of the grid bars using the redeposition milling technique.
After sectioning the bottom part, the volume was transferred to the next grid bar, and this process was repeated until the entire volume was present on the grid in the form of lamellae with an approximate thickness of 4 µm. Following the milling procedure, the lamellae were ready to be used for TEM imaging.
Using this technique, the researchers successfully imaged a large fraction of the cell types and tissues within the same sample. Additionally, they were able to reconstruct a ribosome with 6.9 Å resolution using subtomogram averaging.
Overall, the development of an effective lift-out technique seems to be crucial for studying multicellular organisms and large tissue samples using cryo-ET.
In recent years, large steps have been taken in developing this technique and increasing its throughput, particularly with the introduction of the serial lift-out technique.
Furthermore, by integrating fluorescent light microscopy into the FIB/SEM, and implementing solutions to prevent ice contamination during sample preparation, the lift-out technique can be more easily adopted as part of the standard cryo-ET workflow. Would the next step be to explore the automation of this process?
References
[1] Mahamid, J. et al., Journal of Structural Biology, 192 (2), 262-269 (2015)
[2] Vanhecke, D. et al., Methods in Cell Biology, Academic Press, 88, 151-164 (2008)
[3] Parmenter, C.D. et al., Journal of Microscopy, 28, 157-174 (2021)
[4] Rubino, S. et al., Journal of Structural Biology, 180 (3), 572-576 (2012)
[5] Schaffer, M. et al., Nat. Methods 16, 757–762 (2019)
[6] Schiøtz, O.H. et al., bioRxiv 2023.04.28.538734
[7] Kuba, J. et al., Journal of Microscopy, 281, 112-124 (2019)
[8] Commercially available cryo microgripper (Labtech)
[9] OmniProbe nanomanipulators (Oxford instruments)
[10] Klumpe, S. et al., Microscopy Today, 30 (1), 42-47 (2022)
[11] Schreiber, D.K. et al., Ultramicroscopy, 194, 89-99 (2018)
.png)