Diabetes Type 1 is considered to be one of the most inheritable common diseases [1] and it is estimated that 9% of the population is affected by this life-threatening disease [2]. Despite the intensive research efforts over the past years, the exact causes of the disease are still unknown.
Electron microscopy (EM) is a popular technique for imaging the islets of Langerhans, primarily due to the high spatial resolution it provides. EM, whether it is transmission electron microscopy (TEM) or scanning electron microscopy (SEM), allows the identification of subcellular features. The disadvantage of EM, however, is very significant as well. It takes a long time to acquire images with a good signal-to-noise ratio. Moreover, identifying cells only by their structure, with no knowledge of their function, is extremely laborious and error-prone.
It would be ideal to have a technique that can first rapidly image cells over a large field of view to identify the area of interest based on cellular function, followed by selective high resolution EM imaging at that location This can be achieved by fluorescence microscopy (FM). Hence, a combination of electron microscopy and fluorescence microscopy is a great solution to image cellular function (from FM) in the context of structural information (from EM). This is called Correlative Light and Electron Microscopy (CLEM) and it is a powerful technique to study the relation between structure and function in biology. However, performing CLEM has its own challenges. Constantly switching from one microscope to another is time-consuming and often contaminates the sample. Since the two microscopes have very different fields of view, accurately overlaying the two images is highly non-trivial and prone to bias.. And the sample preparation for the two imaging techniques are not the same, which means that it is not exactly the same sample that is being imaged in FM and EM. All in all, performing CLEM requires skilled operators, research expertise and is very labour-intensive.
We believe that the operation of the microscope should not be the main focus or the limiting factor in performing scientific research. To enable researchers to focus their time and energy on analyzing and interpreting the images rather than in acquiring them, Delmic has developed the Integrated CLEM technique and innovated the world’s first product using Integrated CLEM, called SECOM. The idea of Integrated CLEM is combining FM and EM in one instrument, and SECOM is designed to be retrofitted onto any SEM, enabling the user to perform integrated FM and EM on one instrument and in an automated manner. The SECOM workflow consists of the acquisition of a fluorescence image, followed by the acquisition of a high resolution EM image, followed by high-accuracy automated overlay of the two. The SECOM greatly simplifies and speeds up the CLEM workflow, thereby enabling high quality research in life sciences.
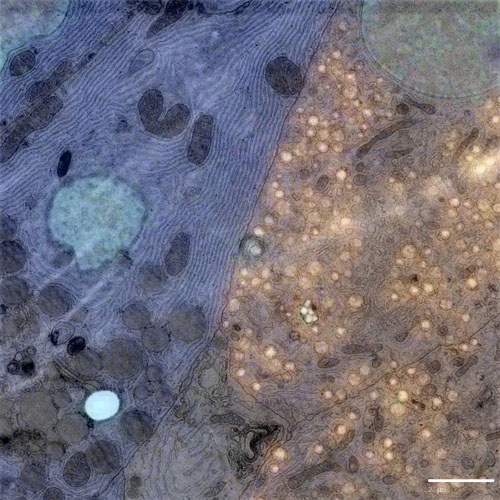
The SECOM platform retrofitted on a Thermo Fisher Verios 460 SEM was used to image beta cells in the Islets of Langerhans in healthy rat pancreas (Figure 1). A customized sample preparation protocol developed for integrated CLEM was successfully implemented, and imaging was performed on a thin section mounted on a conducting glass coverslip using the workflow described.

Figure 1: Simultaneously acquired correlative image of the islets of Langerhans imaged on the Delmic SECOM integrated with a Thermo Fisher Verios 460 Scanning Electron Microscope. Sample courtesy: B.N.G. Giepmans & P. de Boer, UMCG.
As seen from the image, the labeling of insulin-producing beta cells with Alexa Fluor 594 (orange) is clearly visible. The image also shows fluorescence of guanine quadruplexes and nuclei (blue). The ultra-structure of the beta cells and other cell types can be examined in detail using the EM contrast. These results demonstrate the potential of CLEM for studying the islets of Langerhans in detail and finding out the cause of Diabetes Type 1 in the near future.
If you are interested in learning more about this research, we invite you to download the application note: Type 1 diabetes: Imaging insights.
References
[1] Wang, Z., Xie, Z., Lu, Q. et al. Clinic Rev Allerg Immunol (2017) 52: 273. https://doi-org.eur.idm.oclc.org/10.1007/s12016-016-8592-1
[2] G. Danaei et al., The Lancet 378, 9785, 31-40 (2011)
.png)