Cryogenic electron tomography (cryo-ET) is an imaging technique that allows the reconstruction of high-resolution models of protein complexes in their near-native state. In cryo-ET, a sample (e.g. eukaryotic cells grown on an EM grid) is first vitrified, then thinned to the appropriate thickness (100-300 nm) using a Focussed Ion Beam (FIB) inside a Scanning Electron Microscope (SEM). Finally, a series of images is captured with a transmission electron microscope (TEM).
These 2D images are then used to reconstruct a 3D volume, a so-called tomogram. From the tomogram, high-resolution structural models of proteins of interest can be reconstructed. During all these steps the sample must remain vitrified at ~ -160 °C.
There are three main sources of ice contamination in the workflow.
The first source is the deposition of ice crystals on the sample from contaminated liquid nitrogen. Ice crystals form on surfaces close to the liquid nitrogen in humid environments. Common sources are clipping and preparation stations, forceps, dewars, and even large main liquid nitrogen tanks and hoses.
Ice crystals deposited on the sample this way are usually both large crystals that block the field of view (Figure 1a) as well as smaller crystals that are spread out over the sample (Figure 1b). Extensive contamination of a lamella with these crystals renders it unusable for tomogram acquisition.
Common tactics to avoid this form of contamination are: handling samples in low-humidity rooms, wearing face masks to avoid breathing directly on liquid nitrogen-cooled surfaces, and heating vessels and tools before use to remove any moisture from their surface. However, even when taking into account all of these precautions, it is impossible to completely avoid ice crystal formation and some level of liquid nitrogen contamination.
The second source of ice contamination is the growth of ice crystals on the samples by the deposition of water molecules from the environment. When a sample is transported outside of the liquid nitrogen (e.g. from a prep station to the FIB/SEM), water molecules in the air can deposit on the sample.
For this reason, sample transfer outside of liquid nitrogen should be performed in a vacuum. The vacuum reduces the amount of water that can be deposited and also minimizes the chance of the sample getting warm. However, transfer systems usually do not reach a high vacuum, meaning that water molecule deposition still takes place. This results in the growth of hexagonal ice on the sample surface (Figure 1c, asterisks).
To avoid this, speed in sample transfer is crucial, making it not an uncommon sight to see researchers running down the hall from one microscope to the next with a transfer stick in hand.
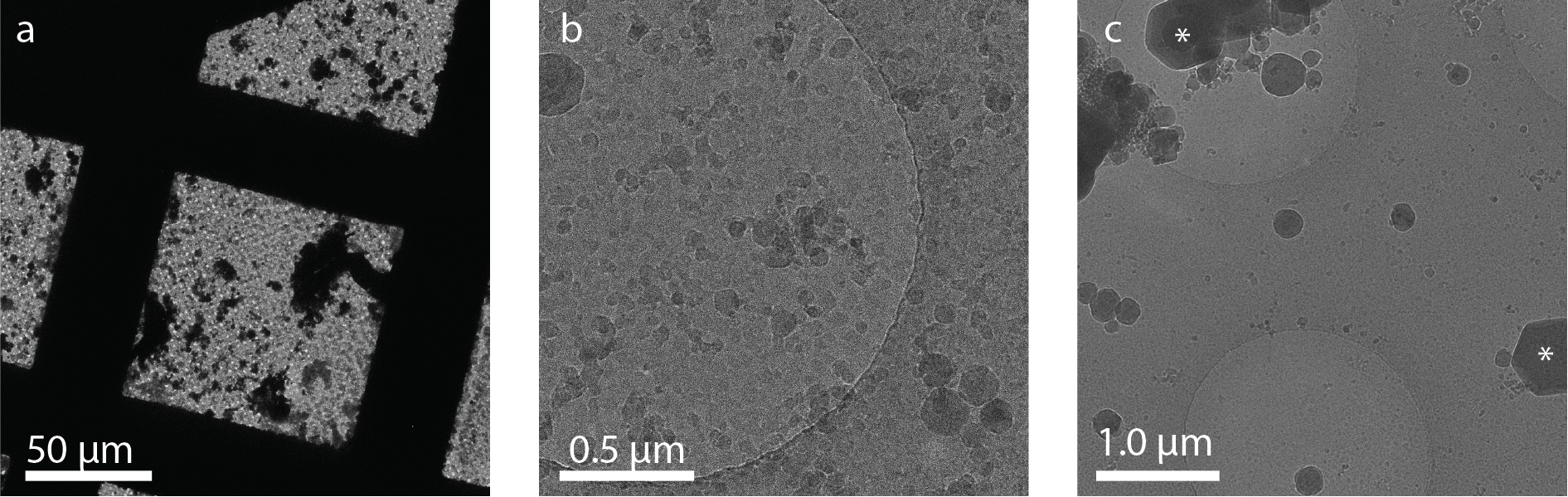 Figure 1. Examples of ice contamination on cryo-EM grids. a) Overview image showing large areas of contamination caused by contaminated liquid nitrogen. b) Higher magnification of the sample from a) shows small ice deposits covering the entire sample. c) Sample contamination with hexagonal ice crystals (asterisks) from water molecule deposition in poor vacuum. Images courtesy: Sebastian Tacke and Stefan Raunser, MPI Molecular Physiology, Dortmund, Germany.
Figure 1. Examples of ice contamination on cryo-EM grids. a) Overview image showing large areas of contamination caused by contaminated liquid nitrogen. b) Higher magnification of the sample from a) shows small ice deposits covering the entire sample. c) Sample contamination with hexagonal ice crystals (asterisks) from water molecule deposition in poor vacuum. Images courtesy: Sebastian Tacke and Stefan Raunser, MPI Molecular Physiology, Dortmund, Germany.
Lastly, ice contamination can even take place in the high vacuum environment of the FIB/SEM. The high vacuum ensures minimal water content, but the low temperature of the sample acts like a “cold trap” that causes gaseous water molecules in the chamber to sublimate into an ice layer when hitting the cold sample.
This process occurs very slowly, but since the milling procedure takes several hours, crystalline ice can build up at rates of around 50 nm/hour at a chamber pressure of approximately 10-7 Bar [1]. With milling times of around 1 hour per lamella, this means that a thin lamella of ~150 nm can be covered with several times its own size in crystalline ice during sample thinning.
When analyzed in the cryo-TEM, this crystalline ice layer on the lamella significantly reduces the quality of the resulting data. Since these ice growth rates are currently accepted by major cryo FIB/SEM suppliers, it puts users under pressure to mill as fast as possible.
To avoid this, it is best to ensure rough milling for all lamella is done first before starting polishing. This reduces the time a finished lamella is exposed to ice contamination from the chamber. Automated milling procedures can also increase efficiency and reduce milling time, leading to less ice build-up. In addition, a higher vacuum in the chamber lowers the amount of water in the chamber, thereby reducing ice growth.
Together, these three sources of sample contamination form an important bottleneck in the current cryo-ET workflow.
At Delmic, we believe that cryo-ET is the best way to understand how the building blocks of life, and that's why we are dedicated to improving, simplifying, and automating the cryo-ET workflow. With METEOR, our integrated fluorescence microscope, you can already significantly reduce sample handling and sample transfer steps, and as a result, minimize ice contamination.
To even further minimize ice contamination at every step of your cryo-EM workflow we developed CERES Ice Defence System. Learn more about it here!
References
[1] S. Tacke et al., “A streamlined workflow for automated cryo focused ion beam milling,” bioRxiv, p. 2020.02.24.963033, Feb. 2020, doi: 10.1101/2020.02.24.963033.
This work is supported by the European SME2 grant № 879673 - Cryo-SECOM Workflow.
.png)