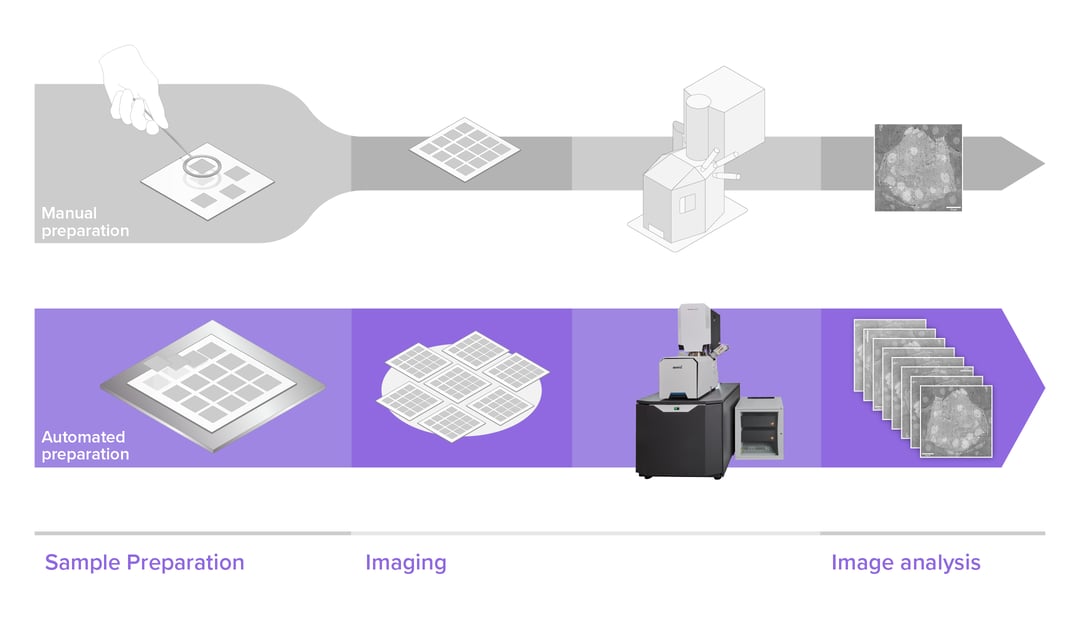
In the past few years electron microscopes have become increasingly faster, increasing their overall throughput. However, this throughput improvement can only be used effectively if sample preparation is able to keep up with the image acquisition. The main bottleneck here lies in the fact that sample preparation requires several time-consuming and repetitive steps to obtain high-quality samples.
Sample staining and embedding
In their native state, biological specimens are incompatible with EM. Exposure to the electron beam and the vacuum required in EMs rapidly render untreated samples too damaged for imaging. Nowadays, a range of sample preparation techniques is available for preparation, which can be fine-tuned for specific research questions or imaging modes. Although reagents and strategies may vary wildly, several basic conditions are addressed for any biological specimen.
Firstly, the specimens should yield a high electron contrast. Heavy metal staining agents, like osmium tetroxide or uranyl acetate are used to improve the visibility of organelles and protein complexes in biological samples. Secondly, samples need to withstand the vacuum inside the microscope. Dehydration with acetone or ethanol, followed by embedding in acrylic or epoxy resin, makes sure that sample ultrastructure is preserved in the vacuum chamber. Thirdly, the sample needs to be thin enough for imaging with EM. Thus, sections are collected from the embedded specimens with an ultramicrotome and transferred onto a substrate to be imaged. Finally, the sample should be conductive to prevent charging by the electron beam, which is usually achieved by coating either the substrate or the sample with a conductive layer of metal or carbon.
Addressing all these steps in sample preparation can quickly become a time-consuming process, requiring time-consuming incubation steps and frequent reagent exchanges. New approaches for sample preparation are therefore continuously examined, with the goal of speeding up sample preparation, and improving image quality.
Sample pickup and placement
Reliable section collection is crucial to maintain high-quality samples for high-throughput EM. Manual serial sectioning is a time-consuming process and mistakes are frequent and costly. Moreover, specimen pickup and placement need to be done in an organized fashion to keep organization of the samples intact. Keeping track of all the individual sections in large volume EM is tricky and challenging. Loss of sections due to contamination, damage or displacement can mean that the sectioning for a project needs to be repeated, as the organelles or structures cannot be followed from one section to the next. In some cases, missing more than one section can already mean that a reconstruction becomes impossible to complete.
Due to the importance of sample preservation, many developments are being made to improve the sample preparation workflow. Automation of sample pickup and placement plays an important role to enhance the throughput of biological specimens during the whole process. Reducing section loss, improving the percentage of useable samples and tracking the large amount of samples are the main goals of these developments.
Role of automation in sample preparation
Automation in sample preparation manifests in a variety of methods, ranging from the initial fixation and embedding, to automated means of section collection. For the former, several solutions have become prominent, such as robotization of reagent exchanges, and automated processing stations able to speed up staining and embedding by microwave heating [1]. These drastically cut down the amount of time and lab work required to obtain a properly fixed and embedded specimen.
Automation of sectioning is another promising development, due to the fragile nature of the sectioning process, which can take months to learn and master. Like with staining and embedding, automation drastically reduces the need for a skilled operator, enabling collection of hundreds or thousands of section with minimal chance of missing a section. Multiple solutions have been proposed, including magnetic [2] and tape-based collection [3].
In MagC, one of these approaches, the process is simplified by combining the sample block with a block loaded with magnetic beads. The two are collected in a single section, meaning that the embedded beads can be used to cluster all sections onto a large substrate, enabling a very high section deposition density for hundreds or even thousands of sections. This process ensures that sections can be cut continually without operator intervention. This was demonstrated in a correlative study of the zebra fish brain, where neuroanatomical tracers were imaged within their ultrastructural volumetric electron microscopy context.
Automation and innovation at Delmic
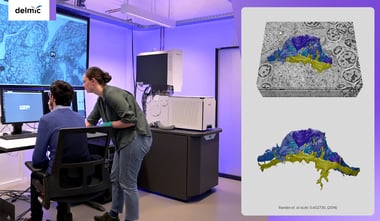
At Delmic we are working on an automated sample preparation workflow to support our FAST-EM system, an ultra-fast multibeam electron microscope. With the increase in imaging speed that EM techniques experience, a faster sample preparation workflow is needed. With the help of the recent developments in automating the preparation of biological samples, high-throughput EM techniques will produce data even faster. This causes a significant shift in research fields that require 3D information of ultrastructures and large volumes, including fields such as nanotomy or connectomics.
Are you interested to know how FAST-EM works and how it could boost your workflow? Watch our recent launch demonstration to see what FAST-EM can do for you.
References
[1] Zechmann, B., & Zellnig, G. (2009). Microwave-assisted rapid plant sample preparation for transmission electron microscopy. Journal of Microscopy, 233(2), 258–268. https://doi.org/10.1111/j.1365-2818.2009.03116.x
[2] Templier, T. (2019). MagC, magnetic collection of ultrathin sections for volumetric correlative light and electron microscopy. ELife, 8, 1. https://doi.org/10.7554/elife.45696
[3] Schalek, R., Kasthuri, N., Hayworth, K., Berger, D., Tapia, J., Morgan, J., Turaga, S., Fagerholm, E., Seung, H., & Lichtman, J. (2011). Development of High-Throughput, High-Resolution 3D Reconstruction of Large-Volume Biological Tissue Using Automated Tape Collection Ultramicrotomy and Scanning Electron Microscopy. Microscopy and Microanalysis, 17(S2), 966–967. https://doi.org/10.1017/s1431927611005708
.png)