Cryo-electron microscopy (cryo-EM) is a general term for performing electron microscopy at cryogenic temperatures. When imaging with an electron microscope, the samples have to be in a high vacuum environment and they are exposed to a high-intensity electron beam. Biological samples are vulnerable to damage and disintegration under such conditions. This problem can be addressed by freezing the samples to cryogenic temperatures, which preserves their structure. Two widely used subtypes of cryo-EM are cryo-ET and SPA (Figure 1).
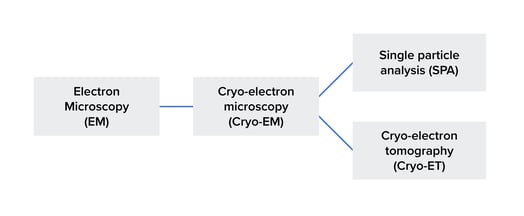 Figure 1: The difference between cryo-EM and cryo-ET. Cryo-EM is a specific type of electron microscopy. SPA and cryo-ET, in turn, are subtypes of cryo-EM. It should be noted that the figure doesn't show all of the techniques in this field.
Figure 1: The difference between cryo-EM and cryo-ET. Cryo-EM is a specific type of electron microscopy. SPA and cryo-ET, in turn, are subtypes of cryo-EM. It should be noted that the figure doesn't show all of the techniques in this field.
Single particle analysis (SPA) is a type of cryo-EM technique that is widely used to generate 3D reconstructions of mostly protein structures. This technique involves imaging samples of the protein of interest in different orientations in 2D (Figure 2). The images of the protein with different orientations are then combined to generate a 3D reconstruction of the protein. To accurately compute the orientations of the protein in the images, a large number of images are required. The presence of symmetry in the protein simplifies this computation [1].
A drawback of SPA is that the protein must be purified prior to imaging, which can be challenging for proteins such as membrane proteins [2]. Additionally, specific protein conformations require stabilization steps during the purification process. Moreover, purified proteins are extracted from their cellular environment, resulting in a loss of information on molecular interactions.
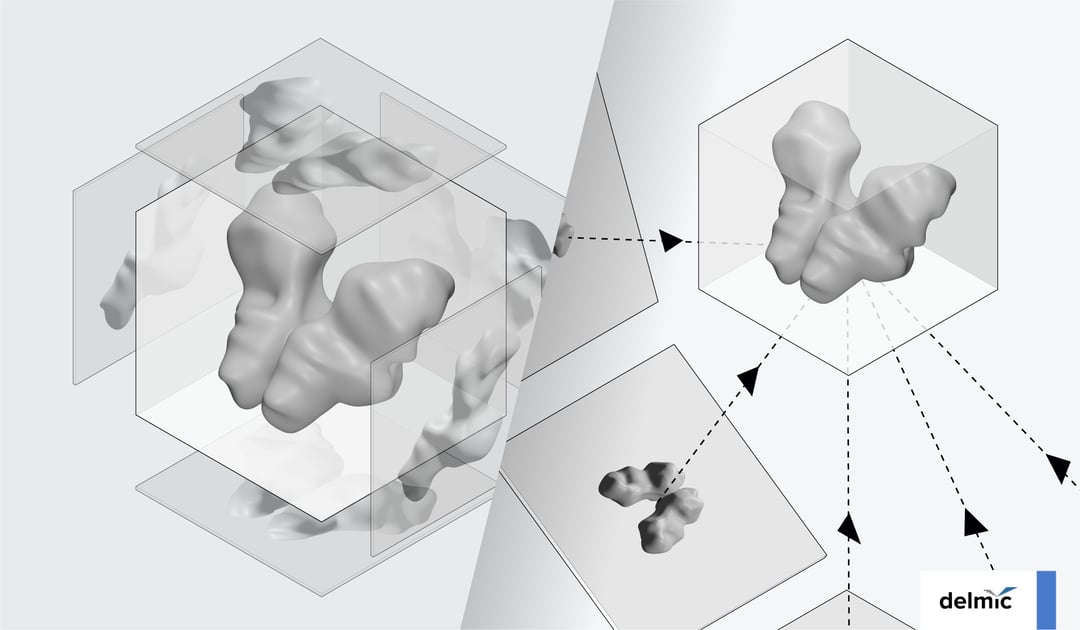
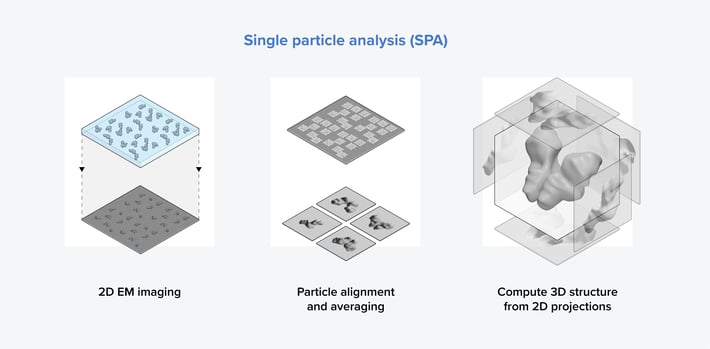 Figure 2: The three main steps in the single particle analysis workflow. First, an image of a sample containing identical purified proteins in various orientations is taken. Subsequently, particle alignment and averaging are conducted to generate 2D images of the protein from different orientations. Finally, the 3D structure of the protein is computed from the 2D images.
Figure 2: The three main steps in the single particle analysis workflow. First, an image of a sample containing identical purified proteins in various orientations is taken. Subsequently, particle alignment and averaging are conducted to generate 2D images of the protein from different orientations. Finally, the 3D structure of the protein is computed from the 2D images.
Cryo-electron tomography (cryo-ET) is another type of cryo-EM that enables researchers to generate 3D reconstructions of macromolecular structures in situ, such as protein complexes, viruses, and organelles. Cryo-ET has an advantage over single-particle analysis (SPA) in that it enables the visualization of macromolecular structures at a nanoscale resolution within their natural environment. This is crucial for gaining insights into their biological function [3].
To obtain a reconstruction of a molecule of interest in situ, most samples have to be thinned first. The most successful technique to prepare a thin section (lamella) within the sample is cryo-focused ion beam milling (FIB), which is performed in a scanning electron microscope (SEM). While thinning, the region of interest can be identified by cryogenic fluorescent light microscopy (cryo-FLM).
After that, the samples can be imaged using a transmission electron microscope (TEM). The sample is tilted from﹣60° to approximately 60°, and 2D images of the molecule are captured at each tilt angle (Figure 3). By combining these images through computational processing, a 3D image or ‘tomogram’ of the molecule can be generated, which can also show the unique features of the molecule. The tomogram can approach near-atomic resolution by using subtomogram averaging, a technique that involves aligning and averaging multiple 3D reconstructions of the molecule of interest.
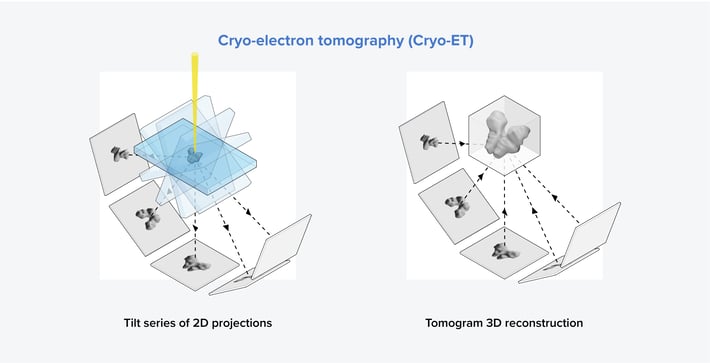 Figure 3: The two main steps in the cryo-ET workflow. First, a sample containing the molecule of interest is tilted from -60° to 60°, and images are captured at each tilt angle. Then, a tomogram can be generated using the images obtained at different tilt angles.
Figure 3: The two main steps in the cryo-ET workflow. First, a sample containing the molecule of interest is tilted from -60° to 60°, and images are captured at each tilt angle. Then, a tomogram can be generated using the images obtained at different tilt angles.
Cryo-EM is thus an umbrella term for electron microscopy techniques including cryo-ET and SPA. These highly advanced techniques allow researchers to view their biological samples at the nanoscale. There are some drawbacks to using cryo-EM, such as the long and tedious workflow, and the risk of ice contamination and damage to the samples. Luckily, solutions to these problems have been developed, such as protection against ice contamination and integrated cryo-FLM. The future of cryo-EM looks cool and bright!
References
[1] J.L. Milne et al., FEBS J., 280 (1), 28-45, (2013)
[2] R. Nygaard et al., Current Opinion in Structural Biology, 64, 26-33, (2020)
[3] M. Turk et al., FEBS Lett., 594, 3243-3261, (2020)
.png)