Cryo-electron tomography (cryo-ET) offers an unprecedented ability to visualize spatial and organizational relationships of biological macromolecules in their native state and environment. Examples of biological structures successfully studied with cryo-ET include viruses (1, 2), cells and their organelles (3), and even tissues and multicellular organisms (4, 5, 6). However, as a cutting-edge technique in its early stages of development, cryo-ET is also highly complex and requires true wizardry to achieve desired results.
The current cryo-ET workflow consists of several phases, starting from sample preparation, followed by imaging under a fluorescence light microscope (FLM), milling in a focused ion beam/scanning electron microscope (FIB/SEM) system, and finally imaging under a transmission electron microscope (TEM). Each of these phases has many steps that can be challenging to execute without an error even for experienced practitioners.
Two major difficulties are present in the cryo-ET workflow: keeping samples vitrified and ice contamination-free, and finding the right region of interest (ROI) in a frozen sample. The ROI can be easily found during and after lamellae milling with our METEOR solution that integrates FLM into the FIB/SEM system. As for the non-vitreous ice build-up, our recent survey with more than 80 participants from cryo-EM labs all over the world shows that sample transfer is the phase in the cryo-ET workflow when most ice contamination appears. Almost half of the respondents have indicated that ice contamination occurs during sample transfer from FLM to the FIB/SEM system and from FIB/SEM to TEM (Figure 1).
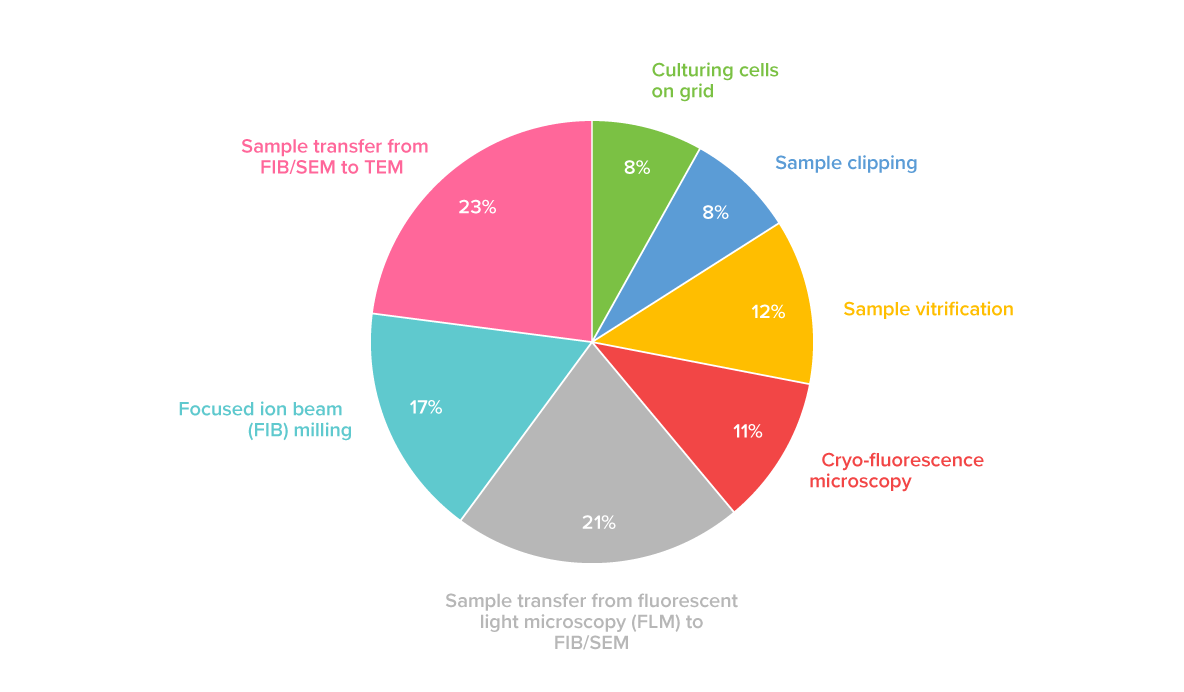 Figure 1. Distribution of responses to a survey question “Where does ice contamination take place?”. The sample transfer steps between FLM, FIB/SEM, and TEM have been indicated as the main sources of ice contamination in the cryo-ET workflow by 44% of the respondents. These results are based on the replies from more than 80 researchers from cryo-EM labs worldwide.
Figure 1. Distribution of responses to a survey question “Where does ice contamination take place?”. The sample transfer steps between FLM, FIB/SEM, and TEM have been indicated as the main sources of ice contamination in the cryo-ET workflow by 44% of the respondents. These results are based on the replies from more than 80 researchers from cryo-EM labs worldwide.
So why does the non-vitreous ice occur during sample transfer in the first place?
In general, ice builds up on a cryogenic sample when water molecules from the vicinity of the sample deposit onto it. After a sample is vitrified, clipped, and placed in a shuttle, it is ready to be transferred, first to FLM, then to FIB/SEM, and finally to TEM. This whole time the sample acts as a cold trap, thus to prevent its devitrification and ice contamination, it must not be exposed to room temperature and air humidity. Providing anhydrous conditions and cooling below the glass transition temperature of the water, are therefore the indispensable factors during sample transfer. The current commercially available sample transfer systems offer low to medium vacuum conditions and only a short time of proper cryogenic temperatures. This puts researchers at risk of finding their precious samples defective or even completely damaged.
We decided to act on this problem and provide a sample transfer system that keeps samples safe and researchers calm. In collaboration with the researchers from Max Planck Institute of Molecular Physiology, we developed the CERES Vitri-Lock system which makes it possible to transfer a cryogenic sample in high vacuum and anhydrous environment, thus protecting the sample from ice build-up. To keep the sample safely vitrified in temperature below -150⁰C we equipped the transfer chamber with active cooling, provided by an external reservoir containing liquid nitrogen. An additional advantage of this design is that the cold surface of the reservoir acts as a cryo pump, maintaining the high vacuum level. The cooling lasts up to 30 min, therefore there is enough time to move the sample between setups without rush and even account for sample screening times, giving researchers more flexibility in the process.
Because of its complex workflow, cryo-ET is tedious and can be frustrating. As one of the cryo-ET researchers tells her students "cry is part of cryo" (7). These pains and the simultaneous huge potential of cryo-ET create an urgent demand for further developments to shorten and simplify the process and so to allow the researchers to reach their full potential and focus on what's important in their work - answering the most fundamental life science questions, pushing the boundaries of our knowledge, and bringing closer cures to diseases.
It is our mission at Delmic to help researchers understand how the building blocks of life work with cryo-ET and we relentlessly try out new ways to streamline this beautiful technique. Check our brochure to learn more on how you can keep your sample safe from ice contamination using CERES Vitri-Lock and other solutions.
References
[1] Vijayakrishnan et al., In situ structure of virus capsids within cell nuclei by correlative light and cryo-electron tomography Sci Rep (2020).
[2] Hangping et al., Molecular Architecture of the SARS-CoV-2 Virus Cell (2020).
[3] Weber et al., Cellular and Structural Studies of Eukaryotic Cells by Cryo-Electron Tomography Cells (2019).
[4] Harapin et al., Structural analysis of multicellular organisms with cryo-electron tomography Nat Methods (2015).
[5] Schaffer et al., A cryo-FIB lift-out technique enables molecular-resolution cryo-ET within native Caenorhabditis elegans tissue Nat Methods (2019).
[6] Gaisin et al., Cryo-Electron Tomography Reveals the Complex Ultrastructural Organization of ulticellular Filamentous Chloroflexota (Chloroflexi) Bacteria Front. Microbiol. (2020).
[7] Marx Calling cell biologists to try cryo-ET Nat Methods (2018).
This work is supported by the European SME2 grant № 879673 - Cryo-SECOM Workflow.
.png)








